二苄叉丙酮的制备
Title: The preparation of Dibenzylideneacetone
Date: Sep 22nd
Name:张瑾瑜
Student id:14321037
Objectives:
1. To prepare Dibenzylideneacetone by Claisen-Schmidt reaction
2. To measure the melting point of purified Dibenzylideneacetone
Principles:
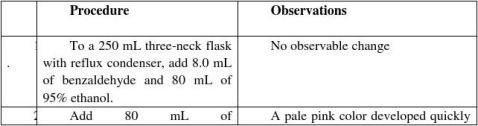
Condensation reaction can be performed at the presence of alkaline as a catalyst to convert Aromatic aldehyde and aldehyde or ketone with α-hydrogen atom to unsaturated α,βaldehyde or ketone. The production rate should be high after dehydration.
Dibenzylideneacetone(dba、C17H14O) can be prepared by an aldol condensation of benzaldehyde and acetone with sodium hydroxide as a catalyst.
Reagents and apparatus
Benzaldehyde, 95% ethanol, 0.5M NaOH(aq) and acetone
250 mL round-bottomed flask, 10 mL pipet, reflux condenser, magnetic stirrer bar, hot-plate stirrer, clamp, suction flask, Buchner funnel
Physical constants
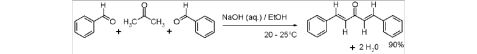
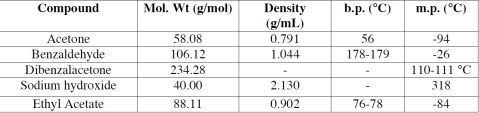
Theoretical mass of product = 2.87 g
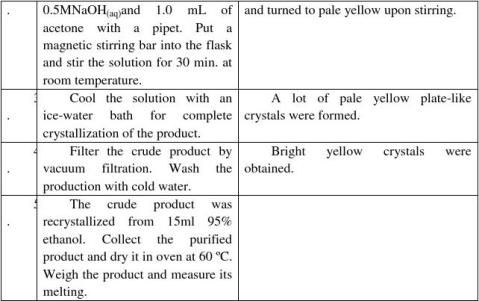
Mass of crude product =
Mass of purified product =
Percent yield = (mass of purified product / theoretical mass of product) x 100% = ? %
Results
Color and shape of the purified product
Melting point of the final product = ?oC ~ ?oC
Answers to questions/ exercise
1.The introduction of extra impurities and the loss of production should be avoided so the solvent should be carefully chosen. Cold water can be used as a washing agent.
2.No, the productivity will not increase. The ratio between acetone andbenzaldehyde has reached the best ratio.
3. If the concentration of alkaline is too high, disproportion reaction of aldehyde will take place.
4. There are cis-cis isomer and cis-trans isomer. Nuclear Magnetic Resonance Spectroscopy can tell the difference.
Discussions
第二篇:实验九 苄叉丙酮和二苄叉丙酮的制备


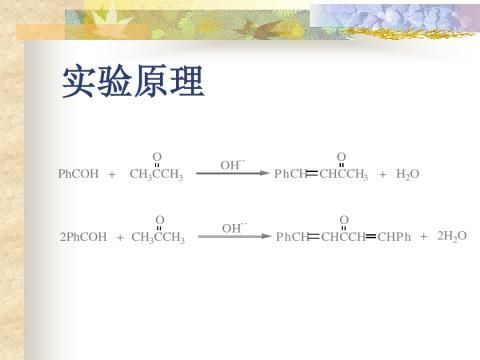




-
二苄叉丙酮的制备
二苄叉丙酮的制备4学时实验目的1学习利用羟醛缩合反应增长碳链的原理和方法2学习利用反应物的投料比控制反应物利用衍生物来鉴别羰基化合…
-
实验二十一 二苄叉丙酮的制备
实验二十一二苄叉丙酮的制备一实验目的1学习利用羟醛缩合反应增长碳链的原理和方法2学习利用反应物的投料比控制反应物利用衍生物来鉴别基…
-
二苄叉丙酮的制备
TitleThepreparationofDibenzylideneacetoneDateSep22ndName张瑾瑜Studen…
-
二苄叉丙酮的制备
实验18二苄叉丙酮的制备4学时一实验目的学习羟醛缩合反应原理和方法学习控制物料比控制反应产物二实验原理ClaisenSchmidt…
-
实验室苄叉丙酮的制备
苄叉丙酮的制备中文名苄叉丙酮中文别名苯丁烯酮苯丁烯1酮3苯丁烯酮苄亚丙酮甲基苯乙烯基甲酮甲基苯乙烯基酮苯亚甲基丙酮甲基肉桂基甲酮乙…
-
苯亚甲基苯乙酮的制备(期末实验报告)
苯亚甲基苯乙酮的制备一实验的目的和要求1掌握羟醛缩合反应的原理和机理学会苯亚甲基苯乙酮的合成方法2掌握水蒸气蒸馏3掌握反应温度控制…
-
实验六_华南师范大学实验报告,纳米二氧化钛太阳能电池的制备及其性能测试
华南师范大学实验报告题目纳米二氧化钛太阳能电池的制备及其性能测试课程密码620xx组别第二组姓名学号20xx2401012指导老师…
- 丙酮二羧酸的制备-毕业设计开题报告
-
实验二十一 二苄叉丙酮的制备
实验二十一二苄叉丙酮的制备一实验目的1学习利用羟醛缩合反应增长碳链的原理和方法2学习利用反应物的投料比控制反应物利用衍生物来鉴别基…
-
二苄叉丙酮的制备
实验18二苄叉丙酮的制备4学时一实验目的学习羟醛缩合反应原理和方法学习控制物料比控制反应产物二实验原理ClaisenSchmidt…