医药相关书目
湖南中医药大学第二届“三湘读书月”活动
推荐书籍目录
一、文学类
1、《读有所得》
2、《诗经》
3、《文心雕龙》
4、《窦娥冤》
5、《乐府诗集》
6、《水浒传》
7、《三国演义》
8、《西游记》
9、《古文观止》
10、《长生殿》
11、《聊斋志异》
12、《儒林外史》
13、《红楼梦》
14、《唐诗·宋词·元曲》
15、《老残游记》
16、《呐喊》
17、《激流三部曲》
18、《子夜》
19、《边城》
20、《骆驼祥子》
21、《雷雨》
22、《围城》
23、《文化苦旅》
24、《红高粱》
25、《中国古代文学作品选》
26、《中国当代文学作品精选》
27、《中国传统文化》
28、《平凡的世界》
29、《黄金时代》
30、《谈美书简》
31、《美的历程》
32、《我的祖国》
33、《恰同学少年》
34、《嘿!青春期》
35、《像雷锋那样》(修订版)
36、《爱的教育》
37.《阅微草堂笔记》
38、《旅行箱的故事》
39、《德兰修女传》
40、《世界因你不同:李开复自传》
41、《念楼学短(一)逝者如斯》
二、哲学类
42、《道德经》
43、《论语》
44、《庄子》
45、《普通逻辑学》
46、《世界思想名著总解说》
47、《中国人文启示录》(第1、2卷)
三、史学类
48、《从文明起源到现代化》
49、《上下五千年》(世界)
50、《希利尔讲世界史》
51、《新编中国文学史》
52、《中国史稿》
53、《社会发展简史》
54、《近代中国与近代文化》
55、《中国哲学史简编》
56、《影响中国历代进程的100件大事》
57、《六十年国事纪要》(文化卷)
58、《唐浩明评点曾国藩家书》(上下)
四、其他类
59、《毛泽东诗词》
60、《毛泽东文选》
(1)纪念白求恩
(2)愚公移山
(3)为人民服务
61、《神七纪实——中国首次太空行走》
62、《10个精彩纷呈的科学争论》(科学案例分析丛书)
63、《论剑:新视野下的中国大战略》
64、《教育的力量》
65、《朱镕基答记者问》
66、《中国艺术》(上、下)
67、《影响中国学生的80部书》
68、《在哈佛听讲座》
69、《学习改变命运》
70、《开国大典》(上下)
湖南中医药大学校权益部
2010-11-23
第二篇:纳米给药系统相关文献
Biomaterials33(2012)1573e1582
ContentslistsavailableatSciVerseScienceDirect
Biomaterials
journalhomepage:www.elsevi

er.com/locate/biomaterials
Gobletcell-targetingnanoparticlesfororalinsulindeliveryandthein?uenceofmucusoninsulintransport
YunJin1,YupinSong1,XiZhu,DanZhou,ChunhuiChen,ZhirongZhang,YuanHuang*
KeyLaboratoryofDrugTargetingandDrugDeliverySystem,MinistryofEducation,WestChinaSchoolofPharmacy,SichuanUniversity,No.17,Block3,SouthernRenminRoad,Chengdu610041,PRChina
articleinfo
Articlehistory:
Received27August2011Accepted27October2011
Availableonline16November2011Keywords:
CSKSSDYQCpeptideGobletcell-targetingMucus
Caco-2/HT29-MTXco-culturedcells
N-trimethylchitosanchloridenanoparticles
abstract
Thepresentstudywastodemonstratetheeffectsofgobletcell-targetingnanoparticlesontheoralabsorptionofinsulininvitro,exvivoandinvivo,andidentifythetargetingmechanismaswellasthein?uenceofmucus.Theinsulinloadednanoparticleswerepreparedusingtrimethylchitosanchloride(TMC)modi?edwithaCSKSSDYQC(CSK)targetingpeptide.Comparedwithunmodi?ednanoparticles,theCSKpeptidemodi?cationcouldfacilitatetheuptakeofnanoparticlesinvilli,enhancethepermeationofdrugsacrosstheepithelium,meanwhile,induceasigni?cantlyhigherinternalizationofdrugsviaclathrinandcaveolaemediatedendocytosisongobletcell-likeHT29-MTXcells.IntransportstudiesacrossCaco-2/HT29-MTXco-culturedcellmonolayer(simulatingintestinalepithelium),theCSKpeptidemodi?cationalsoshowedenhancedtransportability,evenifthetargetingrecognitionwaspartiallyaffectedbymucus.Moreover,itwasfoundtheexistenceofmucuswaspropitioustothetransportofinsulinfrombothmodi?edandunmodi?ednanoparticles.Inthepharmacologicalandpharmacokineticstudiesindiabeticrats,theorallyadministratedCSKpeptidemodi?ednanoparticlesproducedabetterhypoglycemiceffectwitha1.5-foldhigherrelativebioavailabilitycomparedwithunmodi?edones.Inconclusion,CSKpeptidemodi?edTMCnanoparticlesshowedsuf?cienteffectivenessasgobletcell-targetingnanocarriersfororaldeliveryofinsulin.
ó2011ElsevierLtd.Allrightsreserved.
1.Introduction
Oraladministrationofinsulinwouldbebene?cialtodiabeticpatients,asitnotonlyalleviatesthepaincausedbyinjections,but
Abbreviations:CSK,CSKSSDYQC;TJs,tightjunctions;NPs,nanoparticles;TMC,N-trimethylchitosanchloride;INS,insulin;FITC,?uoresceinisothiocyanate;Rho-UEA-I,rhodamine-conjugatedulexeuropaeusagglutininIlectin;MTT,3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazoliumbromide;AMCA-INS,7-amino-4-methy-(3-coumaringlacetic)acid-conjugatedinsulin;EDC$HCl,1-[3-(dimethyla-mino)propyl]-3-ethylaarbodiimidehydrochloride;NHS,N-hydroxysuccinimide;DMEM,dulbecco’smodi?edeaglemedium;CS,chitosan;CH3I,methyliodide;NaOH,sodiumhydroxide;NMP,N-methylpyrrolidone;DQ,degreeofquaterniza-tion;1HNMR,1Hnuclearmagneticresonance;FT-IR,Fouriertransforminfrared;TPP,tripolyphosphate;HCl,hydrochloricacid;HPLC,reverse-phasehighperformanceliquidchromatography;EE,entrapmentef?ciency;PS,physiologicalsaline;PBS,phosphatebuffersolution;PFA,paraformaldehyde;OCT-compound,optimalcuttingtemperature-compound;HEPES,4-(2-hydroxyethyl)-1-piperazineethansulfonicacid;CLSM,confocallaserscanningmicroscope;EDTA,ethylenediaminetetraaceticacid;HBSS,hank’sbalancedsaltsolution;PMSF,phenylmethanesulfonyl?uoride;BCA,bicinchoninicacid;TEER,transepithelialelectricresistance;MW,molecularweight;Papp,apparentpermeabilitycoef?cient.
*Correspondingauthor.Tel./fax:t862885501617.
E-mailaddress:huangyuan0@(Y.Huang).1
The?rsttwoauthorscontributedequallytothis

work.0142-9612/$eseefrontmatteró2011ElsevierLtd.Allrightsreserved.doi:10.1016/j.biomaterials.2011.10.075
canalsomimicthephysiologicalfateofinsulinandmayprovideabetterglucosehomeostasis[1].Nanocarrierisconsideredtobeapromisingvehiclefororaldeliveryofinsulin.Itcanofferdrugprotectionandfacilitatedrugabsorptionthroughtheintestinalmucosa.However,oneofthegreatestchallengesindevelopinganef?cientnanocarrierfororaldeliveryistoovercometheabsorptionbarrierofintestinalmucosa,consistingofintestinalepithelialcellsaswellasmucuslayer[2].
Nanocarriersmodi?edwithspeci?cligandstargetedtothereceptorsonthesurfaceofepithelialcellsareproposedtofacilitatetheoralabsorptionofpeptidesandproteins[2].However,onlylimitedkindsoftargetingagents,suchaslectins,vitaminB12andtransferrin,havebeenreportedtotargettoenterocytesorMcells[3e6].Meanwhile,thefact,beingoftenneglected,isthattherealeffectofepithelialcell-targetingmightbegreatlyaffectedbythemucuslayerpresentingonepithelium.Mucus,secretedbygobletcells,isacomplexandrobustbarrier,whichprotectsepithelialsurfacebyrapidlytrappingandremovingforeignparticles[7],thuscouldserveasaphysicalbarriertothetargetingrecognition.Lectin-conjugatedparticleswerereportedtoshowcross-reactionwithmucusandtheirtargetingef?ciencieswerediminishedbymucus[8].Furthermore,theeffectsofmucuslayeronthemostreported
1574Y.Jinetal./Biomaterials33(2012)1573e1582
targetingnanocarrierswerekeptunknownsincetheinvitroeval-uationsofmostepithelium-targetingsystemswereperformedontheCaco-2cellmodelwhichpossessesnomucus[9e11].Therefore,itisimportanttoexploitmoreeffectivetargetingligandswhichpossesshighspeci?cityforrecognition,soastoprovidesuf?cienttargetingeffectivenesswhileatthesametimeavoidbeingblockedbymucus.Meanwhile,evaluationonthetargetingef?ciencyandmechanismofthetargetingsysteminthepresenceofmucusisconsideredasanotherimportantissue.
Recently,aCSKSSDYQC(CSK)peptideidenti?edfromarandomphage-peptidelibrarythroughaninvivophagedisplaytechniquewasfoundtohaveaf?nitywiththegobletcellwhichisthesecondlargestpopulationofintestinalepithelialcells.ThepeptidecouldfacilitatethetransportofM13bacteriophageacrosstheintestinalepitheliumbyitsgobletcell-targetingproperty[12].ThisstudyimpliesCSKpeptidecouldbeusedasapotentialgobletcell-targetingligandfororaldeliveryofnanocarriers.However,nofurtherresearchwasreported.
Chitosanisacationicpolymer,whichisthesecondmostabundantpolymerinnatureaftercellulose.Itpossessesseveralpropertiessuchasbiocompatibility,biodegradability,permeationenhancingeffectviaopeningthetightjunctions(TJs)betweentheintestinalepithelialcells.Thesepropertiesmakeitasuitablecarriermaterialfororaldeliveryofpeptideandproteindrugs[13].Kisselhascomparedtheuptakeofchitosannanoparticles(NPs),poly-styreneNPsandPLA-PEGNPsbymucus-secretingMTX-E12cells[14].Althoughitwasalsofoundpartiallyboundtomucus,theinternalizedfractionofchitosanNPswasstillhigherthantheothertwotestedNPs,demonstratingthatchitosanNPscouldhavecomparativelyhighercapabilitytopenetratethroughthemucuslayer.Unfortunately,chitosanalwaysbecomesinsolublebyitsdeprotonationmechanismatneutralandbasicpHenvironment,resultinginthedecreaseofabsorptionenhancingability[15].N-trimethylchitosanchloride(TMC),apartiallyquaternizedderivativeofchitosan,ischaracterizedbygoodsolubilityandpermeationenhancingeffectinphysicalpH.Hence,modifyingTMCnanoparticleswithepithelium-targetingligandmightbeaneffec-tivemeansfortheoraldeliveryofpeptidesandproteins.
Therefore,theaimofthepresentstudywastoestablishgobletcell-targetingnanoparticlesusingCSKpeptidemodi?edTMCmaterial(TMC-CSK),evaluatetheireffectivenessasoralinsulin(INS)vehicles,andinvestigatethein?uenceofmucusonthetar-getingeffect.Twointestinalloopligatedmodels(invivoandexvivo)wereusedfortheabsorptionstudiesof?uorescentblankanddrugloadedNPs.Mucus-producingHT29-MTXcellsandtheco-incubatedCaco-2/HT29-MTXcellmodelwhichsimulatedtheepitheliumwereappliedfortheinvitroevaluationsoftargetingef?ciencyandthein?uenceofmucus.Finally,hypoglycemiceffectandrelativebioavailabilityofthetargetingNPsweretestedindiabeticrats.Inall,thegobletcell-targetingnanoparticlesandthein?uenceofmucusontheepithelium-targetingnanocarrierswerestudiedforthe?rsttime.
2.Materialsandmethods2.1.Materialsandanimals
Chitosan(deacetylationdegree>90%andmolecularweightof400kDa)wasprovidedbyAKBiotechCo.,Ltd.(Shandong,China).Porcineinsulin(30IU/mg)waspurchasedfromWanbangBio-ChemicalCo.,Ltd.(Jiangsu,China).CSKSSDYQCpeptidewaschemicallysynthesizedbyKaijieBio-pharmaceuticalsCo.,Ltd.(Sichuan,China).Fluoresceinisothiocyanate(FITC),rhodamine-conjugatedulexeuropaeusagglutininIlectin(Rho-UEA-I)and3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazoliumbromide(MTT)wereallpurchasedfromSigmaeAldrich(St.Louis,MO,USA).N-Acetyl-L-CysteinewasobtainedfromAladdinChemistryCo.,Ltd.(Shanghai,China).7-Amino-4-methy-(3-coumaringlacetic)acid-conjugatedinsulin(AMCA-INS)wassynthesizedbyYoukebiotechCo.,Ltd.(Shanghai,China).
1-[3-(Dimethylamino)propyl]-3-ethylaarbodiimidehydrochloride(EDC$HCl)wasgainedfromMeapeoCo.,Ltd.(Shanghai,China).N-Hydroxysuccinimide(NHS),N-methylpyrrolidone,iodomethaneandacetonitrilewereallobtainedfromKelongchemicalCo.,Ltd(Chengdu,China).Otheragentswereallanalysisgrade.
Caco-2cellsweregainedfrominstituteofBiochemistryandCellBiology(Shanghai,China).HT29-MTXcelllinewasakindgiftfromDr.TheclaLesuf?eur(INSERM,Paris,France).TheywerebothculturedinDulbecco’sModi?edEagleMedium(DMEM)(Gibco,GrandIsland,NY,USA)containing10%fetalcalfserumand1%non-essentialamino(Hyclone,Logan,UT,USA).
MaleSpragueeDawleyratsweighing250?20gweresuppliedbyExperimentalAnimalCenterofSichuanUniversity(protocolnumberforanimalstudy:CSDGZ-10).Theratswerehousedataroomtemperatureof22?2??Candarelativehumidityof50?10%.
2.2.SynthesisofTMCandTMC-CSK
TMCandTMC-CSKweresynthesizedastheschematicroute(SupportinginformationFig.S1).Brie?y,TMCwasobtainedbymethylationofaminegroupsofchitosan(CS)withmethyliodide(CH3I)inastrongbasesolution(sodiumhydroxide,NaOH)usingN-methylpyrrolidone(NMP)assolvents.Thereactionproceededfor45minat60??C.Theproductwaspuri?edbydialysisandthenlyophilized(SNL216V,SavantInstrumentsInc.,NY,USA).Thedegreeofquaternization(DQ)wascalculatedfromtheintegrationof1Hnuclearmagneticresonance(1HNMR,UNITYINOVA-400,VarianInc.,CA,USA)[16].
TheobtainedTMCwassubsequentlyconjugatedwithCSKpeptideviaamidebondformedamongtheresidualprimaryaminogroupsonTMCandcarboxylgroupsonCSKpeptide.TMC(0.27mmolofprimaryaminogroups),EDC$HCl(0.45mmol)andNHS(0.45mmol)weredissolvedin12mlofwater(pH8.0)and?lledwithnitrogen.ThentheCSKpeptidewasaddedata?nalamountof0.045mmol.Thereactionwasconductedatambienttemperaturefor3daysindark.Subsequently,theproductwasdialyzed,lyophilizedandstoredat4??C.TheobtainedTMC-CSKwasidenti?edby1HNMRandFouriertransforminfrared(FT-IR)spectra.Thecontentoftheconjugatedpeptidewasdeterminedthroughtheaminoaciddetection(835-50,HitachiCo.,Tokyo,Japan).
Moreover,FITC-labeledTMCandTMC-CSK(FITC-TMCandFITC-TMC-CSK)werepreparedbasedonthereactionbetweentheisothiocyanategroupsofFITCandtheprimaryaminogroupsofTMCasreported[17].2.3.PreparationofNPs
2.3.1.PreparationofblankNPs
The?uorescentblankNPspreparedusingFITC-TMCandFITC-TMC-CSK(FITC-TMCNPsandFITC-TMC-CSKNPs)werepreparedusingionotropicgelationmethod[18].PluronicF68wasusedasastericstabilizer[19].Brie?y,1mg/mloftripoly-phosphate(TPP)solutioncontainingpluronicF68(0.4%,w/v)wasaddeddrop-wiselytoFITC-TMCorFITC-TMC-CSKsolution(1mg/ml)ataratioof1:5(v/v).Then,thesolutionwasstirredatroomtemperaturefor20min,yieldinganopal-escentsuspension.Thesuspensionwasultracentrifugedat10,000rpmfor30min(AllegraX-22R,BeckmanCoulterInc.,CA,USA).Thesupernatantwasdiscardedandthenanoparticlesformedwereresuspendedforfurtheruse.
2.3.2.PreparationofINS-loadedNPs
Thenon-?uorescentINS-loadedNPspreparedusingTMCandTMC-CSK(TMCINSNPsandTMC-CSKINSNPs)werepreparedasdescribedpreviously[20].Brie?y,INSwasdissolvedinhydrochloricacid(HCl)solution(0.01N,pH2.0)ataconcen-trationof1mg/ml,andthepHwasadjustedto8.0using1MNaOHsolution.Then,theINSsolutionwaspre-mixedwithTPPsolution(1mg/ml)containingpluronicF68(0.4%,w/v)ataratioof1:2.5(v/v).Thepre-mixedsolutionwasaddeddrop-wiselytoTMC-CSKaqueoussolution(1mg/ml)atanequalvolume.Themixturewasstirredatroomtemperaturefor20min,yieldinganopalescentsuspension.Theresultantsuspensionwasultracentrifugedat10,000rpmfor30minat4??C.Thesupernatantwasremovedforthedeterminationofentrapmentef?ciencyandthenanoparticlesformedwereresuspendedforfurtheruse.
Inaddition,fortheobservationandevaluationofINSinthefollowingtests,itwaslabeledwithFITC(FITC-INS)asdescribedinprevioussectionandtheFITC-INSloadedNPspreparedusingTMCandTMC-CSK(TMCFITC-INSNPsandTMC-CSKFITC-INSNPs)wereformedusingtheabove-mentionedmethod.2.4.CharacterizationofNPs
2.4.1.Sizeandzetapotential
TheblankNPsanddrugloadedNPswerecharacterizedfortheirsizeandzetapotentialwithaMalvernZetasizeNanoZS90(MalvernInstrumentsLtd.,Malvern,UK).Allmeasurementswereperformedintriplicate.
2.4.2.Entrapmentef?ciencyofdrugloadedNPs
TheamountoffreeINSinthesupernatantofnon-?uorescencelabeledNPswasmeasuredbyareverse-phasehighperformanceliquidchromatography(HPLC)method(Agilent1200series,CA,USA).SeparationwasachievedonaDiamosilC18
Y.Jinetal./Biomaterials33(2012)1573e1582
1575
column(150mm?4.6mm,5mm)withmobilephaseofacetonitrile-water(28:72,contained0.2MNa2SO4andthepHwasadjustedto2.3withphosphoricacid)andthedetectionwavelengthwassetat214nm[21].
Besides,theamountoffreeFITC-INSinthesupernatantof?uorescence-labeledNPswasmeasuredby?uorospectrophotometer(ShimadzuCorp.,Tokyo,Japan)andtheexcitationandemissionwavelengthsweresetat488and516nm,respectively.
Theentrapmentef?ciency(EE%)ofdrugloadedNPswascalculatedasfollowing[22]:EE%?
totalamountofinsulinàfreeinsulin
totalamountofinsulin
?100
2.4.3.StabilityofINSinNPs
TheactivityofINS-loadedTMCNPswasexaminedbyanHPLCmethodaccordingtotheU.S.Pharmacopeia[23]andthestudiesofHoyerGLandTiyaboonchaiW[24,25].Brie?y,200mg/mlofstandardinsulinwaspreparedin0.01NHClsolution.TMCINSNPscontaining200mgofINSweredissolvedin1.0mlof0.01NHClsolution.Thenthesamplewascentrifugedat10,000rpmfor20minbeforesubjectiontoHPLCdetermination.Thepotencyofinsulinwascalculatedasthefollowingequation:Ct?Cs?Rt=Rs
whereCtstandsforthepotencyoftestsampleandCsisthepotencyofstandardINS.RtispeakareaoftestsampleandRsispeakareaofstandardINS.
2.4.4.Mucinadsorption
ThetwostudiedNPs(TMCINSNPsandTMC-CSKINSNPs)werecentrifugedandresuspendedin5mlofmucinsolution(0.5mg/ml,pH5.5)andincubatedat37??Cfor5h.Thenthedispersionswerecentrifugedandthefreemucincontentinthesupernatantwasmeasuredbyacolorimetricmethodusingperiodicacid/schiffstaining[26].Testswereperformedintriplicateforeachsample.2.5.Absorptionstudiesintheligatedintestinalloops
2.5.1.Absorptionstudiesinvivo
TheinvivouptakeofblankanddrugloadedNPswerequalitativelyevaluatedusingtheligatedintestinalloopsmodelinvivo.AllexperimentswereapprovedbytheInstitutionalAnimalCareandUseCommitteeofSichuanUniversity.MaleSpragueeDawleyratsweighing250?20gwerefastedovernightbeforeexperi-ments,butallowedfreeaccesstowater.Theratwasanesthetizedwithpentobarbitalsodium(0.04mg/kg),andthen2-cmsectionsofileumfromsmallintestinalloopweremadeandwashedwithphysiologicalsaline(PS),thenligatedatbothends.NPssolution(resuspendedinphosphatebuffersolution(PBS),0.2ml)withequal?uo-rescentintensitywasinjectedintotheloop.Atdifferenttimepoints(0.5,1,2and3h),ratwassacri?cedbycervicaldislocationandthesectionofeachloopwasremoved,extensivelywashedusingPBS.ThewashedPBSofblankNPsgroupatthetimeintervalof3hwascollectedforquantitativelymeasurementby?uorospec-trophotometer.Subsequently,theremovedloopsofbothblankanddrugloadedNPsafterwashingateverytimepointwere?xedby4%paraformaldehyde(PFA)for2handimmersedin30%sucroseat4??Covernight.Sampleswerefrozenquicklyinliquidnitrogen-cooledOCT-compound(OptimalCuttingTemperature-compound,‘Tissue-Tek’,MilesLaboratoriesInc.,Indiana,USA).Thesmallintestinaltissue-sectionswerepreparedbyCryotome(HM505E,E-Microm,Zeiss,Walldorf,Germany)in5mmthicknessandputontothegelatin-coatedslideglasses.Thetissue-sectionswerewashedwithdistilledwaterforseveraltimes.MucusdropletsofgobletcellswerelabeledbyRho-UEA-Idilutedin4-(2-Hydroxyethyl)-1-piperazineethansulfonicacid(HEPES)buffer(1:100,v/v)at4??Covernightandwashedwithdistilledwater.Thetissue-sectionswerevisualizedusingconfocallaserscanningmicroscope(CLSM,Live5DUO,CarlZeiss,Jena,Germany)[12,27].2.5.2.AccumulativepermeationstudiesofINS-loadedNPsexvivo
Tofurtherinvestigatethe?nalabsorptionofdrugsfromCSKpeptidemodi?edNPs,FITC-INStransportedacrosstheepithelialmucosaofratileumexvivowasquantitativelymonitoredasdescribedbyYin[28].Brie?y,ratsweresacri?cedand2-cmsectionsofileumloopwerecutout.TMCFITC-INSNPsorTMC-CSKFITC-INSNPs(0.2ml)usedinsection2.5.1wassyringedintotheloop,whichwasincubatedinKreb’-Ringerbufferat37??Cwithsmoothshaking.Ateachtimeintervals(0.5,1,2and3h),theincubationbufferwastakenforthedeterminationofFITC-INSby?uo-rospectrophotometer.Thesamevolumeoffreshbufferwasadded.Alloftheexperimentswereperformedintriplicate.2.6.Cellstudies
2.6.1.Cellculture
Thehumancolonadenocarcinomacells,Caco-2andHT29-MTXcells,werecultivatedseparatelyin75cm2culture?asksusingDMEMsupplementedwith10%fetalbovineserum,1%non-essentialaminoacids,1%L-glutamine,penicillin(100IU/
ml)andstreptomycin(100mg/ml).Bothculturesweremaintainedat37??C,95%relativehumidityand5%CO2.Priortothetest,cellswereharvestedusing0.25%trypsincontaining0.05mMethylenediaminetetraaceticacid(EDTA)anddilutedatadensityof5?104cells/mlforMTTanduptakeassays.Then,thecellsuspensionswererespectivelyseededonto96-wellplates(Corning,NY,USA),andincubatedfor2daysbeforecytotoxicitytestsand7daysbeforeuptakestudies.Fortransportassays,theCaco-2òandHT29-MTXcellsweremixedataratioof1:1andseededontotheTranswellchambersconsistingofpolycarbonatemembrane(0.4mminporesize,0.33cm2ofòcellgrowtharea,Costar)atadensityof3?104cells/well.Then,theTranswellchamberswereplacedinto24-wellplates,intowhichtheDMEMwereadded.Thecellswereallowedtogrowanddifferentiatefor21daysbeforeuse[29].2.6.2.Cytotoxicity
ThecytotoxicityofthesynthesizedpolymerwasevaluatedusingMTTassaywithHT29-MTXcellsandCaco-2cells,respectively.Priortothetest,themediumin96-wellplateswasremoved.Subsequently,thecellswerewashedwithPBSandincu-batedwith100mlofTMCorTMC-CSKsolutionsinHank’sbalancedsaltsolution(HBSS)for3h(concentrationsofTMCandTMC-CSKwere0.125,0.25,0.5,1and2mg/ml,thepHwassetat7.0)andthensubmittedtoMTTassay.HBSSwasusedasreferencefor100%cellviability[28].Testswereperformedintriplicateforeachsample.
2.6.3.UptakestudywithHT29-MTXcells
InordertoestimatethetargetingeffectofCSKpeptidemodi?cationontheinternalizationofTMCFITC-INSNPsinvitroandtherelevantmechanism,uptakestudieswereconductedonthegobletcell-likeHT29-MTXcellsasdescribedprevi-ously[14].
ToremovethemucussecretedontheapicalsideofHT29-MTXcells,thecellswerepre-incubatedwith10mMacetylcysteinedissolvedinHBSSandkeptunderagitationfor60minat37??Cbeforestudy.Subsequently,thecellswererinsedwithHBSSandallowedtoequilibrateat37??Cfor30min,andthenincubatedwith100mlTMCFITC-INSNPsorTMC-CSKFITC-INSNPssuspension(atadoseof220mg/mlofFITC-INS)intransportbuffer(HBSS)at37??C.Toinvestigatethein?uenceofincu-bationtime,thesupernatantswereremovedatpredeterminedtime(0.5,1,2and3h),thenthecellswerewashedtwicewithice-coldPBS.Celllysiswasachievedwithlysisbuffercontaining1%TiritonX-100,50mMHEPES(pH7.5),150mMNaCl,2mMNa3VO4,100mMNaF,100units/mlaprotinin,20mMleupeptinand0.2mg/mlphe-nylmethanesulfonyl?uoride(PMSF)[30].Thenthecellassociated?uorescencesandproteinsweredeterminedbyVarioskanFlash(ThermoFisherScienti?c,MA,USA)andbicinchoninicacid(BCA)assaykit(KeyGenBiotechCo.,Ltd.,Nanjing,China),respectively.TheamountsofuptakewereexpressedasthequantityofFITC-INSassociatedwith1mgofcellularprotein.
WiththepurposeofidentifyingthepossibleinternalizationmechanismofNPsbyHT29-MTXcells,thecellswereincubatedindifferentconditionsortreatedwithspeci?cagents.(I)Tostudytheeffectoftemperatureoncellularuptake,cellswereincubatedwithtestsamplesat4??Cfor3h.(II)Totesttheeffectofsodiumazide,anactivetransportinhibitor,100mMsodiumazidewasdissolvedintheNPssuspensionandincubatedwithcellsfor3hat37??C.(III)Tostudytheeffectofprotaminetreatment,anadsorptive-mediatedendocytosisinhibitor,1mMprotaminesulfatewasdissolvedintheNPssuspensionandincubatedwithcellsfor3hat37??C.(IV)Toinvestigatetheeffectofdesulfurization,cellswerepre-incubatedwith35mMsodiumchlorateincellculturemediumfor48h,rinsedtwicewithtransportbufferandincubatedwiththeNPssuspensionfor3hat37??C[31].(V)TostudythecompetitiveinhibitioneffectoffreeCSKpeptide,cellswerepre-incubatedwithCSKpeptide(0.06mM)inthecellculturemediumfor1h,rinsedtwicewithtransportbufferandincubatedwiththeNPssuspensionfor3hat37??C.(VI,VII)Toexaminetheeffectsofchlorpromazineand?lipin,whichweretheclathrinandcaveolaeendocytosisinhibitors,cellswereincubatedwith10mg/mlchlorpromazineor1mg/ml?lipindissolvedinNPssuspensionandtreatedfor3hat37??C[32].Inaddition,thecontrolsampleswereconductedat37??Cfor3hwithoutanytreatment,andtheresultsoftheinhibitiontestswerepresentedasthepercentageofthatinternalizedincontrol.
2.6.4.Transportthroughtheco-culturedCaco-2/HT29-MTXcellmonolayer
TofurtherinvestigatethetransportofCSKpeptidemodi?edNPsacrosstheepithelialcellsandthein?uenceofmucusonthetargetingrecognition,theco-culturedcellòmonolayerwasincubatedfor21daysafterbeingseededontheTranswellinserts,mimickingtheintestinalepitheliumwithbothepithelialcellsandmucuslayer.Theintegrityofthemonolayerwascheckedusingpropranololwhichismainlyabsorbedbythetranscellularrouteandatenololwhichismainlytransportedthroughtheparacellularpathway[33].
Thein?uenceofincubationtimeontransportwasinvestigated.Priortothestudies,themediumsintheapicalandbasolateralchamberswerereplacedwithpre-warmedHBSS.ThecellswererinsedwithHBSSandallowedtoequilibrateat37??Cfor30min,andthenincubatedwith100mlofTMCFITC-INSNPsorTMC-CSKFITC-INSNPssuspension(220mg/mlofFITC-INS)intransportbufferat37??C.Atthedeterminedtimepoints(0.5,1,2and3h),0.2mlofsamplesweretakenfromthebasolateralchamberandequalvolumesofHBSSweresupplemented.TheamountoftransportedFITC-INSwasdeterminedusingVarioskanFlash,andtheaccumulative
1576Y.Jinetal./Biomaterials33(2012)1573e1582
transportofFITC-INSwascalculated.Simultaneously,thetransepithelialelectricresistance(TEER)valuesweremeasuredwithaMillicell-ERS(Millipore,MA,USA).
Toinvestigatethein?uenceofmucuslayer,thetransportofthetwostudiedNPswerealsoconductedontheco-culturedmodelintheabsenceofmucus(removedusingthemethoddescribedabove).Andforfurtherstudies,transportprocedurewascarriedoutat4??CorwiththeexistenceoffreeCSKpeptideastheinhibitionagent(0.06mM)whichadministered30minbeforetest.
2.7.Pharmacologicalandpharmacokineticalstudies
2.7.1.Diabetesinducing
Diabeteswasinducedinratsbyaninjectionofstreptozotocin(65mg/kg)dis-solvedina10mMcitratebuffer(pH4.5)aspreviouslydescribed[21].Thebloodglucoselevelwasdeterminedusingaglucosemeter(JPS-6,YichengBiotech.Co.Ltd.,Beijing,China).Ratswereconsideredtobediabeticwhentheirfastingglycemiawashigherthan16.0mMoneweekafterstreptozotocintreatment.
2.7.2.Hypoglycemiceffectandrelativebioavailabilitystudiesafteroraladministration
PriortooraldeliveryofdrugloadedNPs,0.5mlofNaHCO3solutionwasintra-gastricallyadministrated.Thendiabeticratswereadministratedwiththefollowingformulations:oraladministrationwithTMC-CSKINSNPs(50.0IU/kgofINS),TMCINSNPs(50.0IU/kgofINS),INSsolution(50.0IU/kg),physiologicalsalineandsubcutaneouslyinjectionwithINSsolution(5.0IU/kg).Bloodsampleswerecollectedfromthetailveinsofratspriortodrugadministrationandatdifferenttimeintervals(1,2,3,4,6,8and10h)afterdosing.Thebloodglucoselevelswerethendetermined.Theareaundertheserumglucoseconcentration-timecurveover10hwascalculatedaccordingtothelineartrapezoidalrule.Thetotaldecreases(D%)inserumwerecalculatedusingamodi?cationmethodasfollows[34]:D%?
AUCephysiologicalsalineTàAUCetestT
AUCephysiologicalsalineT
?100
whereAUCisthetotalareaunderthecurveofplasmaglucoseconcentrationvs.time.
FortheanalysisofplasmaINSlevels,bloodsampleswerecentrifuged(3000rpm,5min)andsubsequentlyquanti?edusinganappropriateinsulinELISAkit(R&DSystem,Inc.,MN,USA).Therelativebioavailability(F%)ofthetestedNPsafteroraladministrationwascalculatedusingthefollowingformula[35].F%?
AUCeoralT?DoseescT
AUCescT?DoseeoralT
?100
whereAUCisthetotalareaunderthecurveofplasmainsulinconcentrationvs.time.
3.Resultsanddiscussion
3.1.Characterizationofsynthesizedpolymers
ChitosanwasconvertedintoTMCtoimproveitswatersolubilityatneutralandbasicpHenvironment.ItwasreportedtheincreasedsolubilityofTMCcanevenbeobservedataDQaslowas10%[36].Consequently,inordertoretainasmuchastheamountoffreeaminogroupstofacilitatethereactionbetweenTMCandCSKpeptide,TMCwithaDQof12.7%waschosen.
TMC-CSKwassynthesizedbycouplingtheaminogroupsofTMCwiththecarboxylgroupsintheC-terminalofCSKpeptide.Andtheself-couplingofCSKpeptidewaspreventedviacontrollingthefeedingmolarratioofTMCandCSKpeptideat6:1.1HNMRandFT-IRspectrawereshowninsupportinginformationFig.S2andS3.Resultsof1HNMRdeterminationhadcon?rmedtheconjugationofTMCwithCSKpeptidebythecharacteristicpeaksat6.752and7.023ppmfortwoprotonsofbenzeneringoftyrosineinCSKpeptidesequence,respectively.TheotherprotonpeaksofCSKpeptiderangedfrom0.9to4.7ppmwereundistinguishedduetotheoverlapofglucoseunitpeaksofTMC.Besides,comparedtoFT-IRspectrumofTMC,thecharacteristicpeakofbenzeneringat798.15cm-1forTMC-CSKwasobserved,againindicatingtheconjugationofCSKpeptidewithTMC.Then,accordingtotheresultofaminoaciddetection,thecontentofCSKpeptideinTMC-CSKpolymerwascalculatedtobe0.09mmol/g.Meanwhile,theFITC-labeledpolymerswerealsocharacterized(datanotshown).
3.2.CharacterizationofNPs
Sinceproteinsareliabletobedegradedandeasilyinactivated,themildionotropicgelationmethodissuitableforthepreparationofproteinloadedNPs[17].
ThecharacteristicsofblankanddrugloadedNPs(includingFITC-labeledNPsandnonFITC-labeledNPs)weresummarizedinTable1.Whencomestothe?uorescence-labeledbatches,thedrugloadedNPspossessedbothlargerparticlesizeandlowerzetapotentialcomparedwithblankbatches,duetotheentrapmentofnegativelychargedFITC-INS.Otherwise,itwasclearthatalltheblankNPsanddrugloadedNPspreparedbyTMC-CSKexhibitedlargersizeandlowerzetapotentialincomparisonwithNPspreparedbyTMC.ThismightbeduetotheintroductionofCSK,apeptidewithmolecularweight(MW)of1018Daandnegativecharge.Moreover,thenon-?uorescence-labeledTMCINSNPsandTMC-CSKINSNPsshowedsizeof318.1?11.5and342.0?5.8nm,respectively,aswellasasimilarEE%.Besides,thehigherEE%ofFITC-INSincorporatedNPs(around87%)comparedwithINS-loadedNPs(around55%)wasprobablyascribedtotheexposednegativechargesendowedbythefreecarboxyandphenolichydroxylgroupsofFITCwhichcouldalsointeractwithTMC.
ThepotencyofstandardINSwas30.0IU/mg.AccordingtotheresultsofHPLCspectra,thepotencyofINSextractedfromtheNPswascalculatedtobe29.30?0.18IU/mg,andtheINSrecoverywas97.68?0.59%.Therefore,itcouldbeconcludedthattheactivityofINSinNPswasmainlyremained,againsuggestingtheionicgela-tionmethodwassuitablefortheincorporationofpeptideandproteindrugs.
Themucinadsorptionwasmeasuredtoevaluatethein?uenceofCSKpeptidemodi?cationonthemucoadhesivepropertiesofTMCINSNPs.TheresultsshowedtheamountofmucinabsorbedbyTMCINSNPswas0.48?0.01mg/ml,while0.35?0.04mg/mlforTMC-CSKINSNPs(P<0.05).SincetheinteractionbetweenmucinandNPswasmainlydeterminedbyparticlesizeandchargeinteraction,theCSKpeptidemodi?edNPswithlargersizeandlowerzetapotentialmayleadtolessabsorptionwithmucin.3.3.Absorptionstudiesinligatedileumloop
3.3.1.TheabsorptionofblankanddrugloadedNPsinvivo
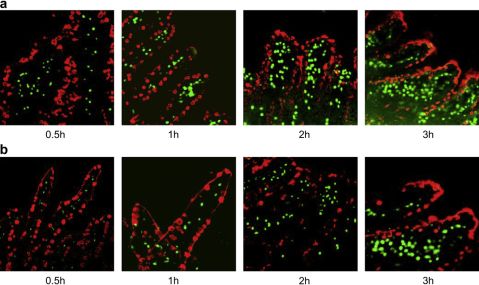
TheabsorptionofNPswasqualitativelyobservedinthevilliofileumloopbyCLSM.Figs.1and2showedtheabsorptionofmaterialsfromblankNPs(FITC-TMC-CSKNPsandFITC-TMCNPs)andFITC-INSfromdrugloadedNPs(TMC-CSKFITC-INSNPsandTMCFITC-INSNPs)atdifferenttimepoints(0.5,1,2and3h),respectively.
Itwasobservedtheabsorptionofallsamplesexhibitedtime-dependentcharacteristicsandthemaximalsignalswerepre-sentedat3h.Modi?cationofbothblankanddrugloadedNPswith
Table1
Characterizationofnanoparticles.NPs
Size(nm)PDI
ZetapotentialEE%(mV)FITC-TMCNPs183.3?6.50.186?0.02210.7?0.3eFITC-TMC-CSK223.1?11.00.179?0.0754.1?0.1e
NPs
TMCFITC-INS298.4?10.40.204?0.0077.0?0.287.5?1.4%NPsTMC-CSK
324.4?10.70.225?0.0213.5?0.388.7?3.1%FITC-INSNPsTMCINSNPs318.1?11.50.215?0.0144.3?0.456.9?2.6%TMC-CSKINS342.0?5.8
0.217?0.022
3.0?0.1
55.4?2.7%
NPs
Y.Jinetal./Biomaterials33(2012)1573e15821577
Fig.1.LocalizationofblankNPspreparedusingFITC-TMC-CSK(a)andFITC-TMC(b)invilliofileumat0.5,1,2and3h.Red?uorescencereferstothemucusdropletsofgobletcells,andgreen?uorescencereferstoFITC.(Forinterpretationofthereferencestocolourinthis?gurelegend,thereaderisreferredtothewebversionofthis
article.)
CSKpeptidecouldpromotetheirabsorptionbyvilliateachtime
point,comparedwithunmodi?edNPs,suggestingthelatent
absorptionenhancingabilityofCSKpeptidemodi?cation.Inter-
estingly,althoughenterocytesarequiteoutnumberedgobletcells
inepithelium,mostoftheintensiveFITC-TMC-CSKsignals(green)
wereassociatedwiththelocationofgobletcells(red)andtheoverlaydisplayedyellowcolor,whereasFITC-TMCsignalsweredispersedlydistributed(magni?edpicturesinFig.1).Thisman-ifestedthepossiblegobletcell-targetingeffectofCSKpeptidemodi?edNPswhichfacilitatedtheabsorption.Ontheotherhand,Fig.2exhibitedthattheFITC-INSpermeateddeeplyintovilli,indicatingtheirwellabsorptioninvivo
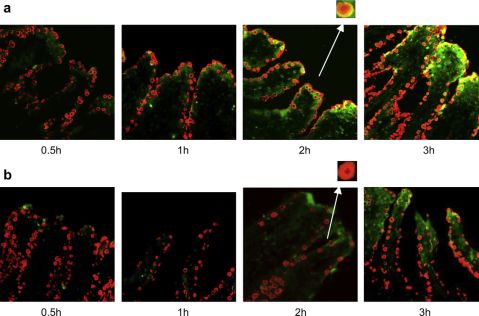
.
Fig.2.LocalizationofFITC-INSloadedNPspreparedusingTMC-CSK(a)andTMC(b)invilliofileumat0.5,1,2and3h.Red?uorescencereferstothemucusdropletsofgobletcells,andgreen?uorescencereferstoFITC.(Forinterpretationofthereferencestocolourinthis?gurelegend,thereaderisreferredtothewebversionofthisarticle.)
1578Y.Jinetal./Biomaterials33(2012)1573e1582
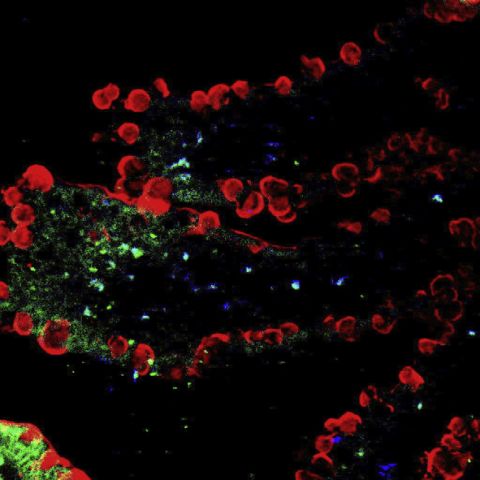
TogetavisualizationofthedistributionofTMC-CSK(material)andINS(drug)invilli,double?uorescentTMC-CSKINSNPspreparedwithAMCA-labeledINS(blue)andFITC-labeledTMC-CSK(green)wereusedininvivouptakestudy.AsshowninFig.3,thebluesignalsofINSpermeateddeeplyintovilli,suggestedthedrugswerewellabsorbed,whilethegreensignalsofTMC-CSKwereonlypresentedinthedistalendandinsideedgeofvilli.ItcouldbeinterpretedthataftertheTJsoftheepitheliumwereopened,INSwithMWofonly5800Dawasmorepriortopassthroughtheintestinalepithelialcellsbyparacellularandtranscellularpath-ways,hencepermeateddeeplyintovilli,whereasTMC-CSKmate-rialswithpositivechargeswere?xedabundantlyonthenegativelychargedinteriorsitesofTJsofepithelialcells[2].
Afterinjectedintoloop,nanoparticlesmayundergo:(1)remainintheintestinallumen;(2)adheretomucin?berandtrappedinmucus;(3)penetratethroughthemucuslayerforpossibleentrytotheunderlyingepithelial[37,38].Tofurtherverifytheaboveexperiments,theamountsofNPsremainedinlumenandtrappedinmucusweremeasuredforinvestigation.TheresultsshowedthattheamountofFITC-TMCNPsremainedinlumenandtrappedinmucuswas2.2-folderhigherthanFITC-TMC-CSKNPs.Inotherword,theamountofFITC-TMC-CSKNPspenetratedthroughthemucuslayerwashigherthantheunmodi?edones,againimplyingthebetterabsorptionpropertyoftargetingNPsacrossthemucuslayerendowedbyCSKpeptidemodi?cation.
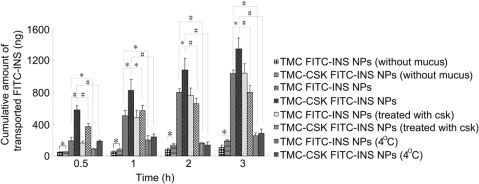
3.3.2.Theaccumulativetransportofdrugsexvivo
ThetransportofNPsacrossintestinalepitheliumintothebloodistheultimatedestination.Hence,theaccumulatedINSpermeationexvivoinligatedileumloopwasconductedtofurtherinvestigatethepermeationabilityofTMC-CSKFITC-INSNPsacrosstheepithelium.ComparedtoTMCFITC-INSNPs,TMC-CSKFITC-INSNPsenhancedtheaccumulativeamountofFITC-INSpermeatedthroughratileum(SupportinginformationFig.S4).ThepermeatedFITC-INSfromCSKpeptidemodi?edNPsat3hwas74.87?10.45ng,whichwas1.7-foldhigherthanthatofunmodi?edNPs(44.91?0.70ng,P<0.05).Theresultsrevealedtheabsorptionenhancingabilityof
Fig.3.DistributionofAMCA-INSandFITC-TMC-CSKofdrugloadedNPsinvilliofileum.Red?uorescencereferstothemucusdropletsofgobletcells,green?uorescencereferstoFITC-TMC-CSK,andblue?uorescencereferstoAMCA-INS.(Forinterpretationofthereferencestocolourinthis?gurelegend,thereaderisreferredtothewebversionofthis
article.)
CSKpeptidemodi?edNPsacrosstheepitheliumassignedbytheirtargetingeffect.
3.4.Cytotoxicityevaluationsofpolymers
Toevaluatethesafetyofthesesynthesizedpolymers,cellularviabilityofHT29-MTXandCaco-2cellsweretestedseparatelyusingMTTassay.BothTMCandTMC-CSKexhibiteddose-dependentcytotoxicityfrom0.125to2mg/mlforHT29-MTXandCaco-2cellsinMTTassay(SupportinginformationFig.S5).Besides,theresultssuggestedthatthemodi?cationofTMCwithCSKpeptideledtoadecreaseincytotoxicity,whichmightbeascribedtotheshieldingofpositivechargesonTMCbythenegativechargesofCSKpeptide.
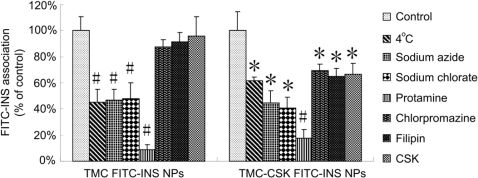
3.5.UptakestudyanditsmechanisminHT29-MTXcells
TheresultsofinvivoandexvivoligatedintestinalmodeltestsshowedthepotentialtargetofCSKpeptidetogobletcellswhereasitsspeci?ctargetingroutewasstillunknownaccordingtoKang[12].Therefore,thecellularuptakeandthepossiblemechanismsforCSKpeptidemodi?edandunmodi?edTMCFITC-INSNPswereinvestigatedwithHT29-MTXcellswhichcompriseapproximately80%maturegobletcellswithTJsinmonolayer.
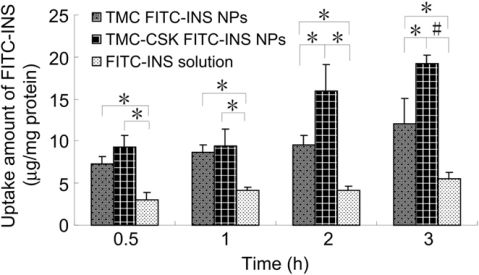
TheamountsofFITC-INSinternalizedbyHT29-MTXcellsatdifferentincubationtimeswereshowninFig.4.TheuptakeofFITC-INSexhibitedtime-dependentpropertiesforallthetestsamples.TMC-CSKFITC-INSNPssigni?cantlyimprovedtheinternalizationofdrugsto15.95?3.15and19.18?0.99mg/mgproteinat2and3h,respectively,whichweresigni?cantlyhigherthanthoseofTMCFITC-INSNPs(9.51?1.21and12.11?3.02mg/mgproteinat2hand3h,P<0.05).TheresultsindicatedtheCSKpeptidemodi?cationcouldgreatlyenhanceHT29-MTXcellsinternalizationofTMCNPs.Themechanismsweresubsequentlyinvestigatedunderdifferentconditions.AsshowninFig.5,theuptakeofFITC-INSinHT29-MTXcellswassigni?cantlyreducedat4??CorwiththeadditionofsodiumazideforTMCFITC-INSNPs(P<0.001)andTMC-CSKFITC-INSNPs(P<0.05),bothofwhichcouldblocktheactivetransportprocesses[14].Besides,theadsorptive-mediatedendocytosisinhibitor,protamine,ledtothestrongestdecreasedinternalizationto8.84?3.80%and17.41?6.93%forunmodi?edandmodi?edNPs,respectively(P<0.001),whichmightbeduetothecompetitionofadsorptiveendocytosis[39].Theseresultsdemonstratedthattheactivetransportprocesses,involvingadsorptiveendocytosis,mightplayanimportantroleontheuptakeofbothNPs.Meanwhile,theinternalizationofthetwo
NPs
Fig.4.UptakeofTMCFITC-INSNPs,TMC-CSKFITC-INSNPsandFITC-INSsolutionbyHT29-MTXcellsatdifferentincubationtime(Mean?SD,n?3e5).*:P<0.05,#:P<0.001.
Y.Jinetal./Biomaterials33(2012)1573e15821579
Fig.5.UptakeofTMCFITC-INSNPsandTMC-CSKFITC-INSNPsbyHT29-MTXcellsatdifferentconditions(Mean?SD,n?3e5).Signi?cantdifferencefromcontrol.*:P<0.05,#:P<
0.001.
decreasedmarkedlywiththepretreatmentofsodiumchlorate,aninhibitorofglycosaminoglycansulfation,whichcouldaffecttheelectrostaticinteractionsbetweenpositivechargedTMCandthenegativechargedglycocalyxontheapicalmembrane,thusin?u-encetheinternalizationofcells[14].
However,theadditionsofchlorpromazineand?lipinwerefoundtoinhibittheuptakeofTMC-CSKFITC-INSNPsto69.5?4.94%and65.16?6.01%inHT29-MTXcells(P<0.05),whiletheunmodi?edNPswerealmostunaffected.Chlorpromazineataconcentrationof6e10mg/mlcoulddisrupttheassemblyanddisassemblyofclathrinand?lipinataconcentrationof1mg/mlisknowntodisruptcaveolaestructurebybindingtocholesterolanddisorganizingthecaveolin[40].Hence,theseresultsimpliedthattheincreasedinternalizationofdrugsfromCSKpeptidemodi?edNPs,comparedwithunmodi?edones,mightbemediatedbycla-thrinandcaveolaeendocytosis.
Furthermore,theadditionoffreeCSKpeptidealsoinhibitedtheinternalizationofTMC-CSKFITC-INSNPs,whereasTMCFITC-INSNPswaskeptunin?uenced,furtherprovingtheexistenceofreceptorsonHT29-MTXcells.
Fromalltheresults,itcouldbeconcludedthattheCSKpeptidereceptormayexistonHT29-MTXcells.Themechanisminvestiga-tionsrevealedthatbesideoftheactivetransportprocessesandelectrostaticinteractionmediateduptakeofbothNPs,theenhancedinternalizationofCSKpeptidemodi?edNPsthanunmodi?edNPsmaybeinvolvedinbothclathrinandcaveolaemediatedendocytosis.
3.6.Transportstudyanditsmechanism
TheabsorptionofINSintocirculatorysystemmeansthatINStransportsacrossintestinalepithelialcellsintothebloodstream.Inotherwords,thepermeatedINSfromtheapicalside,eitherthroughthetranscellularwayortheparacellularway,shouldbereleasedanddeliveredtothebasolateralsideofmonolayers.
Therefore,inourstudy,theco-culturedcellmodelconsistingofbothabsorptiveenterocyte-likeCaco-2cellsandthemucus-producingHT29-MTXcellswereappliedforthe?rsttimetoeval-uatethetransportofTMCnanoparticles.Thisco-culturedmodelcouldbettersimulatetheintestinalepitheliumbyvirtueofnotonlythepresenceofmucuslayer,butalsoaTEERvalueclosetointestinalepithelium[29].Itseemstobeasuitableinvitromodeltoevaluatethepermeabilityoforaldeliveredvehiclesaswellasthein?uenceofmucus.Theapparentpermeabilitycoef?cient(Papp)ofatenololandpropranololfortheco-culturedcellmodelwere2.46?10à6and1.10?10à5cmsà1,respectively,whichimpliedtheintegrityofthecellmonolayer[29,41].Meanwhile,onlythecellmonolayerswithTEERvalueswithintherangeof300e450Ucm2wereused.
ThecumulativetransportedFITC-INSindifferentconditionswaspresentedinFig.6.Thetransportexhibitedtime-dependentandtemperature-dependentproperties.TheamountsofFITC-INSpermeatedthroughCaco-2/HT29-MTXcellmonolayerweregreatlyincreasedwiththemodi?cationofCSKpeptidewithorwithoutthepresenceofmucusateverytimepoint(P<0.05).Besides,thepermeationofFITC-INSfromCSKpeptidemodi?ed
NPs
Fig.6.TransportstudiesofTMCFITC-INSNPsandTMC-CSKFITC-INSNPsacrosstheco-incubatedCaco-2/HT29-MTXmonolayeratdifferentconditions(Mean?SD,n?3e5).*:P<0.05,#:P<0.001.
1580Y.Jinetal./Biomaterials33(2012)1573e1582
Fig.7.BloodglucoselevelsindiabeticratsfollowingoraladministrationofTMCINSNPsandTMC-CSKINSNPsataninsulindoseof50IU/kg(Mean?SD,n?4).Signi?cantdifferencefromINSsolution(Sol)(*)andfromTMCINSNPs(#):P<0.05.
inthepresenceofmucuswereinhibitedwiththeadditionoffreeCSKpeptideateachtimeinterval(P<0.05),whileTMCFITC-INSNPsremainedunaffected.
Inaddition,thetwostudiedNPsexhibitedasimilareffectonthereductionofTEERvalue(about50%reduction)oftheCaco-2/HT29-MTXcellmonolayerinthepresenceofmucus.However,itisknownthattheabilityofTMCNPstoreduceTEERvaluedependsonparticlezetapotential[42]anditwasworthnoticingthatCSKpeptidemodi?edNPsexhibitedaninferiorzetapotentialthanunmodi?edNPs(asshowninTable1).ThepossibleexplanationforthesimilareffectonthereductionofTEERvaluemightowntothehigheramountofCSKpeptidemodi?edNPs?ltratedthroughthemucusandaccumulatedontheapicalsideofcells,asmentionedinsection3.3.1,whichcouldcounteracttheinferiorzetapotentialofsinglenanoparticleandsubsequentlyfacilitatethepermeationofFITC-INS.Thisresultwasalsocon?rmedbythefactthatthetransportofCSKpeptidemodi?edNPswasremarkablysuppressedbytheadditionoffreeCSKpeptidewhichmightinducecompetitiveinhibition.
ThemechanismofabsorptionenhancingabilityofTMCNPswasreportedtobeacombinationofmucoadhesionandtransientopeningofTJsonthemucosalcellmembrane[43].TheionicattractionbetweenTMCandmucuscouldincreasethedrugconcentrationintheextracellularenvironmentwhichisfavorabletothedrugpermeation.Nevertheless,themucusmayalsorestricttheTMCNPs’abilityofopeningTJsbypreventingthemfromreachingcellsurfaces[44].SincetherewereindeedsomedivergentopinionsabouttheeffectofmucusonTMCNPsinpreviouslyliteratures[14,45,46],andthein?uenceofmucusonthetargetingeffectandrecognitionwasrarelyinvestigatedforthereportedepithelium-targetingligands,itwouldbehighlysigni?canttoclarifythein?uenceofmucusonthebehaviorofCSKpeptidemodi?edTMCNPs.
Inourstudies,itwasveryinterestingthatthetransportedamountofFITC-INSwasmuchhigherwiththepresenceofmucusforbothmodi?edandunmodi?edNPsateverytimeinterval(Fig.6),whichwasconsistentwiththereportofKissel[14].Theresultwasquitedifferentfromtheobservationsinpreviousstudies,inwhichthemucuslayeractedasadiffusionbarrierforNPs[46].However,itshouldbenotedthatinthesereports,Caco-2cellsandHT29cellswereseparatelyusedasmucus-freeandmucus-secretingcellmodels.Infact,HT29cellsnotonlyprovidedamucuslayerbutalsocouldresultinadistinctpermeationbehaviorbecauseitisadifferentcelltypefromCaco-2cells.
Therefore,inourtests,thecoherentcellmodelswereusedforthe?rsttimetoinvestigatethein?uenceofthemucusinthetrans-portstudiesbypreservingorremovingthemucusfromthecellmodel.
AnotherissueshouldbestressedthatthepermeationenhancingratioofCSKpeptidemodi?cation(theamountofpermeatedFITC-INSinCSKpeptidemodi?edNPsgroupversusthatoftheunmod-i?edNPsgroup)increasedfrom1.15to1.75during0.5e3hintheabsenceofmucus,whiledecreasedfrom2.98to1.30inthepres-enceofmucus.Itcouldbeinterpretedthat,intheabsenceofmucus,theincreasedpermeationenhancingratiomightbemediatedbythepredominantactiveroleofCSKpeptidemodi?cationduringthewholetransportprocess.Bycontrast,whenitcametothesituationwiththepresenceofmucus,thehighlypermeationenhancingratioat0.5h(2.98)mightbeduetothetargetingmodi?cation.However,withtheincreaseofincubationtime,theover-trappingeffectofmucuswasgraduallydominated,whichledtothedecreasedpermeationenhancingratio.Eventhough,signi?cantlyincreasedtransportofTMC-CSKFITC-INSNPswasstillobservedcomparedwithTMCFITC-INSNPsinthepresenceofmucusateverytimepoint(P<0.05),implyingthefeasibilityandeffectivenessofCSKpeptidemodi?cationonTMCNPs.
Ingeneral,CSKpeptidemodi?cationofnanoparticlescanenhancethedrugpermeationbytheirtargetingproperty,althoughitcouldbepartiallyin?uencedbymucuslayer.
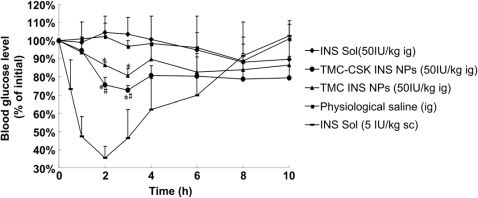
3.7.HypoglycemiceffectandrelativebioavailabilityofdrugloadedNPs
ThepharmacologicaleffectsofTMC-CSKINSNPsandTMCINSNPswereevaluatedondiabeticratsafteroraladministration.AsshowninFig.7,bothTMC-CSKINSNPsandTMCINSNPsexhibitedrelativelystronghypoglycemiceffects(P<0.05)ascomparedwithinsulinsolutionat2hand3hpost-administration.Moreover,withthemodi?cationofCSKpeptide,TMC-CSKINSNPsshowedabetterhypoglycemiceffectwiththemaximalbloodglucosedepressionof28%at3h,comparedtoTMCINSNPs(20%,p<0.05).Furthermore,tointuitivelyelucidatethehypoglycemiceffectofCSKpeptidemodi?edNPs,theD%ofeachformulationwascalculated.ItwasobtainedthattheD%ofCSKpeptidemodi?edNPswere1.52-foldand33.90-foldhigherthannon-modi?edNPsandINSsolutions,suggestingimprovedpharmacologicaleffectbyCSKpeptidemodi?cation.Duringthisexperiment,thefastingbloodglucosedidnotreturntotheinitiallevelafter10h,sameasother
previous
Y.Jinetal./Biomaterials33(2012)1573e15821581
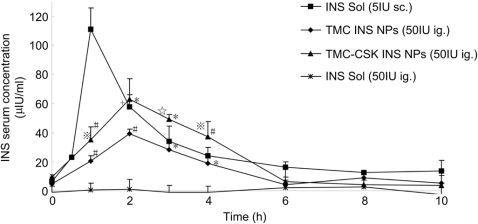
Fig.8.PlasmainsulinlevelindiabeticratsfollowingoraladministrationofTMCINSNPs,TMC-CSKINSNPs,INSsolution(Sol)(50IU/kg)andsubcutaneousinjectionofINSSol.(5.0IU/kg)aspositivecontrol(Mean?SD,n?4).Signi?cantdifferencefromINSSol:(*),P<0.001;(#),P<0.01.Signi?cantdifferencefromTMCINSNPs:(t),P<0.001;(q),P<0.01and(※),P<0.05.
Table2
Pharmacokineticparametersofinsulinindiabeticratsafterintragastricadministration(i.g.)ofTMC-CSKINSNPs,TMCINSNPs,INSsolutionandsubcutaneous(s.c.)administrationofINSsolution(n?4).
INSsolution(s.c.)
Dose(IU/kg)AUC(mg/L*h)Cmax(mIU/mL)Tmax(h)FR%
5.0
407.96?63.64110.91?14.721.0
100.00%
TMC-CSKINSNPs(i.g.)50.0
230.87?41.2362.87?3.362.05.66%
TMCINSNPs(i.g.)50.0
150.69?27.5639.57?2.642.03.69%
INSsolution(i.g.)50.0
16.10?32.794.01?5.065.30.39%
Cmax:maximumplasmaconcentration;Tmax:timeatwhichCmaxisattained;FR:relative
bioavailability.
reports,whichmightbetheconsequenceofthedualeffectsofhungerandhypoglycemicagents.
Furthermore,apharmacokineticanalysiswascarriedouttodeterminetherelativebioavailabilityofinsulinfromNPs.Fig.8showedtheplasmainsulinlevelsfollowingintragastricadminis-tration(i.g.)ofTMC-CSKINSNPs,TMCINSNPsandinsulinsolution,aswellassubcutaneousinjection(s.c.)ofinsulinsolution.ThepharmacokineticparameterswerelistedinTable2.BothTMC-CSKINSNPsandTMCINSNPsexhibitedsigni?canthigherplasmainsulinconcentrationcomparedwithinsulinsolutionat1e4hpost-administration(P<0.01).Furthermore,thehighestplasmainsulinconcentrationafteroraladministrationofTMC-CSKINSNPs(62.87?3.36mIU/mL)waspresentedat2h,whichwassigni?cantlyhigherthanthatofTMCINSNPs(39.57?2.64mIU/mL)(P<0.001).Inaddition,theAUCvalueoftheCSKpeptidemodi?edNPswas230.87?41.23mg/L*h,whichwas1.53-foldhigherthanunmodi-?edones(150.69?27.56mg/L*h).Finally,therelativebioavail-abilityofTMC-CSKINSNPs(5.66%)was14.5-foldand1.5-foldhigherthanthatofinsulinsolution(0.39%)andTMCINSNPs(3.69%),respectively.
Overall,theinvivopharmacologicalandpharmacokineticef?-caciesshowedgoodcorrelationswiththeimprovedabsorptionandtransportofINSintheligatedintestinalloopandcellstudiesinvitro.TheresultsofrelativebioavailabilityclearlyrevealedthatTMCNPsandTMC-CSKNPscouldpartiallyprotectINSfromenzymaticdegradationthroughthegastricintestinal(GI)tracttransitandfacilitateitsabsorptionbyepithelium.Moreover,themodi?cationwithCSKpeptidewouldfurtherimprovetheabsorptionofTMCNPsasanoraldeliveredvehicleforINS,whichwasveri?edtoberesultedbygobletcell-targetingef?ciency.Otherwise,tofurtherimprovethebioavailabilityoforaldeliveredINS,somestrategiessuchas
increasingthestabilityofTMCNPsorovercomingtheexcessivetrapofparticlesbymucuslayerareunderinvestigation.4.Conclusions
Inthepresentwork,gobletcell-targetingTMCnanoparticleswerepreparedandcharacterized.TheCSKpeptidemodi?cationcouldeitherpromotetheuptakeofnanoparticlesinvilliorenhancethepermeationofdrugsacrosstheepitheliumthanunmodi?edNPs.Meanwhile,comparedwithTMCFITC-INSNPs,theinternali-zationofdrugsfromTMC-CSKFITC-INSNPswassigni?cantlyhigherinHT29-MTXcells,whichwascon?rmedtoberesultedbyclathrinandcaveolaemediatedendocytosis.Inthetransportstudiesacrosstheco-culturedCaco-2/HT29-MTXcellmodel,CSKpeptidealsoshowedexcellenttransportenhancingabilityasatargetingagent,althoughthetargetrecognitionofCSKpeptidemodi?edNPscouldbepartiallyin?uencedbymucus.Moreover,theexistenceofmucuswaspropitioustothetransportofinsulinfrombothmodi?edandunmodi?ednanoparticles.Atlast,thehypoglycemiceffectandtherelativebioactivityofTMC-CSKINSNPsweresigni?cantlyimprovedbymodi?cationofCSKpeptide,suggestingthatCSKpeptidewasapotentgobletcell-targetingagentandcouldbeusedasaprom-isingligandfororaldeliveryofpeptidesandproteins.Acknowledgments
Wegratefullyacknowledge?nancialsupportfromtheNationalNaturalScienceFoundationofChina(81173010),agrantfromtheNationalS&TMajorProjectofChina(2009ZX09310-002)andYouthacademicleaderFoundationofSichuanprovince(09ZQ026-051).
1582Y.Jinetal./Biomaterials33(2012)1573e1582
Appendix.Supplementarymaterial
Supplementarymaterialassociatedwiththisarticlecanbefound,intheonlineversion,atdoi:10.1016/j.biomaterials.2011.10.075.References
[1]KhanGhilzaiNM.Newdevelopmentsininsulindelivery.DrugDevIndPharm
2003;29:253e65.
[2]RogerE,LagarceF,GarcionE,BenoitJP.Biopharmaceuticalparametersto
considerinordertoalterthefateofnanocarriersafteroraldelivery.Nano-medicine(Lond)2010;5:287e306.
[3]VandammeK,MelkebeekV,CoxE,DeforceD,LenoirJ,AdriaensE,etal.
In?uenceofreactionmediumduringsynthesisofgantrezAN119nano-particlesfororalvaccination.EurJPharmBiopharm2010;74:202e8.
[4]LochnerN,PittnerF,WirthM,GaborF.Wheatgermagglutininbindstothe
epidermalgrowthfactorreceptorofarti?cialCaco-2membranesasdetectedbysilvernanoparticleenhanced?uorescence.PharmRes2003;20:833e9.[5]ChalasaniKB,Russell-JonesGJ,JainAK,DiwanPV,JainSK.Effectiveoral
deliveryofinsulininanimalmodelsusingvitaminB12-coateddextrannanoparticles.JControlRelease2007;122:141e50.
[6]KavimandanNJ,LosiE,PeppasNA.Noveldeliverysystembasedon
complexationhydrogelsasdeliveryvehiclesforinsulin-transferrinconju-gates.Biomaterials2006;27:3846e54.
[7]KnowlesMR,BoucherRC.Mucusclearanceasaprimaryinnatedefense
mechanismformammalianairways.JClinInvest2002;109:571e7.
[8]LaiSK,WangYY,HanesJ.Mucus-penetratingnanoparticlesfordrugandgene
deliverytomucosaltissues.AdvDrugDelivRev2009;61:158e71.
[9]KadiyalaI,LooY,RoyK,RiceJ,LeongKW.Transportofchitosan-DNAnano-particlesinhumanintestinalM-cellmodelversusnormalintestinalenter-ocytes.EurJPharmSci2010;39:103e9.
[10]ChalasaniKB,Russell-JonesGJ,YandrapuSK,DiwanPV,JainSK.Anovel
vitaminB12-nanosphereconjugatecarriersystemforperoraldeliveryofinsulin.JControlRelease2007;117:421e9.
[11]AndersonKE,StevensonBR,RogersJA.Folicacid-PEO-labeledliposomesto
improvegastrointestinalabsorptionofencapsulatedagents.JControlRelease1999;60:189e98.
[12]KangSK,WooJH,KimMK,WooSS,ChoiJH,LeeHG,etal.Identi?cationof
apeptidesequencethatimprovestransportofmacromoleculesacrosstheintestinalmucosalbarriertargetinggobletcells.JBiotechnol2008;135:210e6.[13]PatelMP,PatelRR,PatelJK.Chitosanmediatedtargeteddrugdeliverysystem:
areview.JPharmPharmSci2010;13:536e57.
[14]BehrensI,PenaAI,AlonsoMJ,KisselT.Comparativeuptakestudiesofbio-adhesiveandnon-bioadhesivenanoparticlesinhumanintestinalcelllinesandrats:theeffectofmucusonparticleadsorptionandtransport.PharmRes2002;19:1185e93.
[15]ChenF,ZhangZR,YuanF,QinX,WangM,HuangY.Invitroandinvivostudy
ofn-trimethylchitosannanoparticlesfororalproteindelivery.IntJPharm2008;349:226e33.
[16]SievalAB,ThanouM,KotzéAF,VerhoefJC,BursseeJ,JungingerHE.Prepara-tionandNMRcharacterizationofhighlysubstitutedn-trimethylchitosanchloride.CarbohydrPolym1998;36:157e65.
[17]WangS,JiangT,MaM,HuY,ZhangJ.Preparationandevaluationofanti-neuroexcitationpeptide(ANEP)loadedn-trimethylchitosanchloridenano-particlesforbrain-targeting.IntJPharm2010;386:249e55.
[18]JulienneMC,AlonsoMJ,GóMezAmozaJL,BenoitJP.Preparationofpoly(D,
L-lactide/glycolide)nanoparticlesofcontrolledparticlesizedistribution:applicationofexperimentaldesigns.DrugDevIndPharm1992;18:1063e77.[19]MüllerRH,RungeSA.Biodegradationofsolidlipidnanoparticlesasafunction
oflipaseincubationtime.IntJPharm1996;144:115e21.
[20]SadeghiAM,DorkooshFA,AvadiMR,SaadatP,Ra?ee-TehraniM,
JungingerHE.Preparation,characterizationandantibacterialactivitiesofchitosan,n-trimethylchitosan(TMC)andn-diethylmethylchitosan(DEMC)nanoparticlesloadedwithinsulinusingboththeionotropicgelationandpolyelectrolytecomplexationmethods.IntJPharm2008;355:299e306.
[21]LiuJ,GongT,FuH,WangC,WangX,ChenQ,etal.Solidlipidnanoparticlesfor
pulmonarydeliveryofinsulin.IntJPharm2008;356:333e44.
[22]ChenF,ZhangZR,HuangY.Evaluationandmodi?cationofn-trimethylchi-tosanchloridenanoparticlesasproteincarriers.IntJPharm2007;336:166e73.
[23]U.S.Pharmacopeia/NationalFormulary(USP/NF).EditedbytheUnitedStates
PharmacopeialConvention.USP34,2011.
[24]HoyerGL,NolanJrPE,LeDouxJH,MooreLA.Selectivestability-indicating
high-performanceliquidchromatographicassayforrecombinanthumanregularinsulin.JChromatogrA1995;699:383e8.
[25]TiyaboonchaiW,WoiszwilloJ,SimsRC,MiddaughCR.Insulincontaining
polyethylenimine-dextransulfatenanoparticles.IntJPharm2003;255:139e51.
[26]HeP,DavisSS,IllumL.Invitroevaluationofthemucoadhesivepropertiesof
chitosanmicrospheres.IntJPharm1998;166:75e88.
[27]PrimardC,RochereauN,LucianiE,GeninC,DelairT,PaulS,etal.Traf?c
ofpoly(lacticacid)nanoparticulatevaccinevehiclefromintestinalmucustosub-epithelialimmunecompetentcells.Biomaterials2010;31:6060e8.
[28]YinL,DingJ,HeC,CuiL,TangC,YinC.Drugpermeabilityandmucoadhesion
propertiesofthiolatedtrimethylchitosannanoparticlesinoralinsulindelivery.Biomaterials2009;30:5671e700.
[29]HilgendorfC,Spahn-LangguthH,RegardhCG,LipkaE,AmidonGL,LangguthP.
Caco-2versusCaco-2/HT29-MTXco-culturedcelllines:permeabilitiesviadiffusion,inside-andoutside-directedcarrier-mediatedtransport.JPharmSci2000;89:63e75.
[30]ChenC,ShenG,HebbarV,HuR,OwuorED,KongAN.Epigallocatechin-3-gallate-inducedstresssignalsinHT-29humancolonadenocarcinomacells.Carcinogenesis2003;24:1369e78.
[31]MislickKA,BaldeschwielerJD.Evidencefortheroleofproteoglycanincation-mediatedgenetransfer.ProcNatlAcadSciUSA1996;93:12349e54.
[32]RejmanJ,BragonziA,ConeseM.Roleofclathrin-andcaveolae-mediated
endocytosisingenetransfermediatedbylipo-andpolyplexes.MolTher2005;12:468e74.
[33]WalterE,JanichS,RoesslerBJ,Hil?ngerJM,AmidonGL.HT29-MTX/Caco-2
coculturesasaninvitromodelfortheintestinalepithelium:invitro-invivocorrelationwithpermeabilitydatafromratsandhumans.JPharmSci1996;85:1070e6.
[34]FanY,LiX,ZhouY,FanC,WangX,HuangY,etal.Improvedintestinaldelivery
ofsalmoncalcitoninbywater-in-oilmicroemulsions.IntJPharm;2011.doi:10.1016/j.ijpharm.2011.06.029.
[35]SonajeK,LinKJ,WeySP,LinCK,YehTH,NguyenHN,etal.Biodistribution,
pharmacodynamicsandpharmacokineticsofinsulinanaloguesinaratmodel:oraldeliveryusingpH-responsivenanoparticlesvs.subcutaneousinjection.Biomaterials2010;31:6849e58.
[36]KotzéAF,LuessenHL,deLeeuwBJ,deBoerAG,VerhoefJC,JungingerHE.
Comparisonoftheeffectofdifferentchitosansaltsandn-trimethylchitosanchlorideonthepermeabilityofintestinalepithelialcells(Caco-2).JControlRelease1998;51:35e46.
[37]Galindo-RodriguezSA,AllemannE,FessiH,DoelkerE.Polymericnano-particlesfororaldeliveryofdrugsandvaccines:acriticalevaluationofinvivostudies.CritRevTherDrugCarrierSyst2005;22:419e64.
[38]PonchelG,IracheJ.Speci?candnon-speci?cbioadhesiveparticulatesystems
fororaldeliverytothegastrointestinaltract.AdvDrugDelivRev1998;34:191e219.
[39]SaiY,KajitaM,TamaiI,WakamaJ,WakamiyaT,TsujiA.Adsorptive-mediated
endocytosisofabasicpeptideinenterocyte-likeCaco-2cells.AmJPhysiol1998;275:514e20.
[40]HuangM,MaZ,KhorE,LimLY.UptakeofFITC-chitosannanoparticlesby
A549cells.PharmRes2001;19:1488e94.
[41]ArturssonP,KarlssonJ.Correlationbetweenoraldrugabsortioninhumanand
apparentdrugpermeabilitycoef?cientinhumanintestinalepitheliamCaco-2cells.BiochemBiophysResCommun1991;175:880e5.
[42]SadeghiAM,DorkooshFA,AvadiMR,WeinholdM,BayatA,DelieF,etal.
Permeationenhancereffectofchitosanandchitosanderivatives:comparisonofformulationsassolublepolymersandnanoparticulatesystemsoninsulinabsorptioninCaco-2cells.EurJPharmBiopharm2008;70:270e8.
[43]SchipperNG,OlssonS,HoogstraateJA,deBoerAG,VarumKM,ArturssonP.
Chitosansasabsorptionenhancersforpoorlyabsorbabledrugs:2.mechanismofabsorptionenhancement.PharmRes1997;14:923e9.
[44]BowmanK,LeongKW.Chitosannanoparticlesfororaldrugandgenedelivery.
IntJNanomedicine2006;1:117e28.
[45]WoitiskiCB,SarmentoB,CarvalhoRA,NeufeldRJ,VeigaF.Facilitatednano-scaledeliveryofinsulinacrossintestinalmembranemodels.IntJPharm2011;412:123e31.
[46]SchipperNG,VarumKM,StenbergP,OcklindG,Lennern?sH,
ArturssonP.Chitosansasabsorptionenhancersofpoorlyabsorbabledrugs.3:in?uenceofmucusonabsorptionenhancement.EurJPharmSci1999;8:335e43.
-
医院宣传标语和口号
医院宣传标语和口号1勇挑重担发奋图强2只要献出一点爱生命因你而精彩3爱心传递生命4以我们的热心关心细心耐心让病人舒心放心安心欢心二…
-
食品药品安全宣传标语口号100条
食品药品安全宣传标语口号100条1国以民为本民以食为天食以安为先2食品安全是金百姓健康是福3食品质量安全责任重于泰山4保障食品安全…
-
中医宣传活动口号
中医中药中国行宣传口号活动口号1发展中医利国利民2发展中医药健康你我他3传承祖国医学造福人类健康4弘扬传统文化服务大众健康5传播中…
-
医院优质服务口号
医院优质服务口号医院优质服务口号从开始到结束我们关注着您的点点滴滴爱的责任责任是一种胸怀象阳光普照万物象大海包容和承载责任又不只是…
-
医院服务口号
医院服务口号一点微笑您能赢得一份真情给我一份信任还您一身健康来到医院安心祝大家身体健康弘扬白求恩精神铸塑医院新形象树医院文明新风展…
-
小学精细化管理总结
认真真做好每件小事,不折不扣地抓好常规落实,一丝不苟地抓严每个细节。一、抓好常规管理,落实日常行为检查。为规范学生行为,组织学生学…
-
五年级一班健康教育总结
张永儿童少年的健康关系到我国的兴衰和民族的未来,要提高民族的健康水平,就必须从小学生做起,使学校成为育人园地。校医是第一位教师,培…
-
11级助残工作总结 2
11级助残小分队工作总结结合我院青年志愿者协会实际情况,我们秉承“奉献、友爱、互助、进步”的志愿服务宗旨,20xx年至20xx年青…
-
小学语文教学经验总结
语文阅读教学带给我的启示以往的阅读教学在一定程度上偏离了正确的轨道。主要表现在:没有摆正教师、学生、文本之间的关系。教师是主宰,主…
-
培训总结模板
用友软件股份有限公司地址:电话:传真:项目培训总结模板建立日期:20xx年x月x日修改日期:20xx年x月x日目录1、2、3、4、…