一定物质的量浓度溶液的配制实验报告
一定物质的量浓度溶液的配制实验报告
班级: 姓名:
实验目的:1、练习配制一定物质的量浓度的溶液。
2、加深对物质的量浓度概念的理解。
3、练习容量瓶、胶头滴管的使用方法。
实验用品:烧杯、容量瓶(100mL)、胶头滴管、量筒、玻璃棒、药匙、滤纸、托盘天平、NaCl(s)、蒸馏水。
实验内容:配制100mL1.00 mol/L的NaCl溶液
(一)实验步骤:
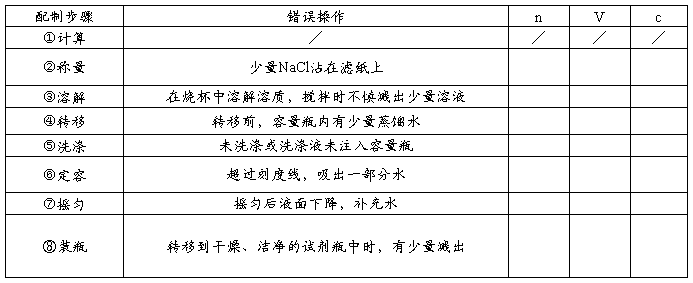
1、计算:需要NaCl固体的质量为 g。
2、称量:用托盘天平称量时,称量NaCl固体的质量为 g。
3、溶解:把称好的NaCl固体放入 中,用量筒量取 ml蒸馏水溶解。
4、移液:待溶液 后,将烧杯中的溶液用 引流注入容量瓶中。
5、洗涤:用少量蒸馏水洗涤烧杯内壁 次,洗涤液也都注入容量瓶。轻轻晃动容量瓶,使溶液混合均匀。
6、定容:将蒸馏水注入容量瓶,液面离容量瓶颈刻度线下 时,改用 滴加蒸馏水至 。
7、摇匀:盖好瓶塞,反复上下颠倒, 。
8、装瓶:将配制好的试剂倒入试剂瓶。
(二)误差分析
第二篇:实验报告
配制一定溶质质量分数的溶液 班级: 姓名: 合作者: 时间:
【实验目的】:
1、初步学会配制一定溶质质量分数的溶液。
2、 能正确使用托盘天平和量筒使用等基本实验操作,学会误差分析。
3、通过配制溶液实验,进行科学严谨的实验态度。
【实验用品】:药品:氯化钠,蒸馏水
仪器: 、 、 (10ml、100ml)、 、 、 。
【探究过程】: 1、配制80g10%的氯化钠溶液
步骤:(1) 计算 :氯化钠的质量 g,水的质量 g 。 (2)称量:用 称出氯化钠 的质量 g ;用 量出水的体积 ml。
(3 )溶解 :把称好的氯化钠倒入干燥的烧杯中,再加入量好的水的体积,用玻璃棒充分搅拌,直到氯化钠全部溶解。
(4)装瓶贴标签 :把配制好氯化钠溶液装入试剂瓶中,盖好瓶塞,贴上标签(注明药品名称和溶质质量分数),放入试剂柜
2、分析影响溶质质量分数
(1)小组讨论:你认为本小组配制的质量分数准确吗?什么原因造成的? 、 、
小结:质量分数偏小的原因:__________________ 、 _________________、______________、 _________________ 等、
质量分数偏大的原因: ____________________________ 。
无影响的: ________________________________________。
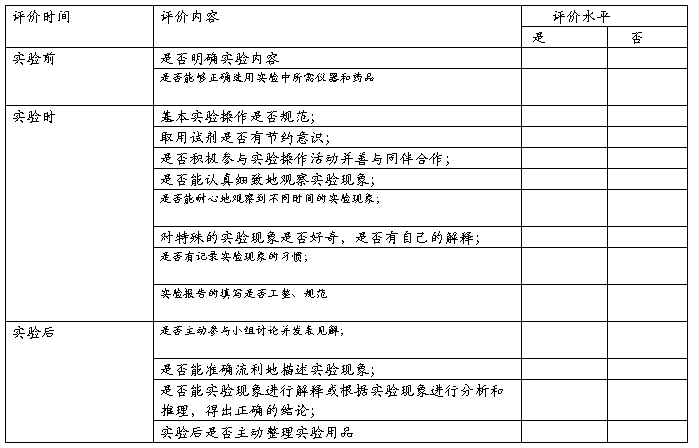
小组实验互评量表
- 配制溶液实验报告
-
一定物质量浓度溶液的配制实验报告
配制一定物质的量浓度的溶液考试内容配制100ml01molLNa2CO3溶液实验名称配制100ml01molLNa2CO3溶液实验…
-
配制一定物质的量浓度的溶液实验报告
配制一定物质的量浓度的溶液实验报告实验目的1练习配制一定物质的量浓度的溶液2加深对物质的量浓度概念的理解3练习容量瓶胶头滴管的使用…
-
一定物质的量浓度溶液的配制实验报告
一定物质的量浓度溶液的配制实验报告班级姓名座号评分实验目的123实验仪器实验药品NaCl实验4配制100mL100molL的NaC…
-
无机化学实验第四版实验三:溶液的配制实验报告
实验名称溶液的配制实验日期温度气压一实验目的1学习比重计移液管容量瓶的使用方法2掌握溶液的质量分数质量摩尔浓度物质的量浓度等一般配…
-
氯化钠溶液的配置实验报告
实验4配制100mL100molL的NaCl溶液班级姓名实验目的1练习配制一定物质的量浓度的溶液2加深对物质的量浓度概念的理解3练…
-
实验报告_酸碱标准溶液的配制和标定
大学化学实验实验一酸碱标准溶液的配制和标定实验目的1掌握标准溶液的配制方法2掌握滴定法定量测定溶液浓度的原理熟悉滴定管移液管的准备…
-
EDTA标准溶液的配制与标定实验报告
EDTA标准溶液的配制与标定EDTA标准溶液的配制与标定一实验目的1掌握EDTA标准溶液的配制与标定方法2掌握铬黑T指示剂的应用条…
- 配制溶液实验报告
-
配制一定物质的量浓度的溶液实验报告
配制一定物质的量浓度的溶液实验报告实验目的1练习配制一定物质的量浓度的溶液2加深对物质的量浓度概念的理解3练习容量瓶胶头滴管的使用…
-
一定物质量浓度溶液的配制实验报告
配制一定物质的量浓度的溶液考试内容配制100ml01molLNa2CO3溶液实验名称配制100ml01molLNa2CO3溶液实验…