实验室质控改善方案
实验室质控改善方案
一、前言
6月18日我公司送水样(南玻项目水样)外检,外检项目包括铁、铜、锌、总磷和氨氮共计5个项目。根据广州工业微生物检测中心的检测报告,铁、铜、锌和总磷的分析结果和我公司实验室分析结果相差非常大,基于第三方检测公司的权威和可靠性,为了提高我公司实验室检测结果的准确度,特提出以下实验室质控改善方案。
二、质控改善方案
1、 实验室现状
1.1 铁、铜、锌、总磷等分析项目制作工作曲线时使用分析纯试剂配制标准溶液,而国家标准分析方法的要求标准溶液配制需用优级纯以上的试剂;
1.2 工作曲线没有定期校准和重新制作,有的工作曲线使用时间已达1年以上。分光光度计长时间使用后,其工作性能会出现一定的波动,这对被测溶液的吸光度会产生一定的影响。另外,使用不同批次试剂也会对同一浓度被测样品的吸光度产生影响。
1.3 样品抽检频率,每2个月一次,偏差率在13.9%~25%之间波动,远大于实验室质控不大于5%的偏差要求。
2、改善方案
2.1 购买铜、锌标准溶液(规格:1000ug/ml,50mg/瓶)、磷酸氢二钾(优级纯)和硫酸铁铵(优级纯)配制铜、锌、磷酸根和铁离子的标准溶液,重新制作工作曲线;
2.2 定期(2月/次)配制已知浓度的质控样进行分析,及时检验分析结果的准确度;控制分析结果偏差在5%以内;
2.3 在排除其他因素干扰的情况下(如加入的试剂过期、样品预处理不当、分光光度计工作是否正常等),根据质控样的偏差值大小确定是否需要重新制作工作曲线 :
偏差值<5%,OK! 偏差值>5% ,重新制作工作曲线。
2.4 工作曲线使用时间最长要求不超过6个月,即每使用6个月需重新制作一次。
2.5 实验室配制试剂需标注配制日期和有效期等,使用前先确定试剂是否在保质期内。若试剂过期,则需重新配制。
2.6固定一些比色管或者容量瓶用于分析同一的指标,可避免其他试剂带来的干扰
2.7 定期对实验人员进行理论考试,加强实验员的理论知识。
3、实验室常规项目质控要求一览表

技术部
20##年7月3日
第二篇:实验室质控
CCA-13276;NoofPages9
ClinicaChimicaActaxxx(2013)xxx–xxx
ContentslistsavailableatScienceDirect
ClinicaChimicaActa
journalhomepage:/locate/clinchim
Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:AninternationalpilotstudyintheframeworkoftheCalibration2000project
RobJansena,?,NutharJassamb,AnnetteThomasc,CarmenPerichd,PilarFernandez-Called,AnaPaulaFariae,HelenaCorreiae,JulianH.Barthf,CasWeykampa,ChristaCobbaertg,MarcThelenh,CarmenRicósd
a
DutchFoundationforQualityAssessmentinMedicalLaboratories(SKML),Nijmegen,TheNetherlandsDepartmentofClinicalBiochemistry,HarrogateDistrictFoundationTrust,Harrogate,UKc
WEQASQualityLaboratory,CardiffandValeUniversityHealthBoard,Cardiff,UKd
SpanishSocietyofClinicalChemistryandMolecularPathology(SEQC),AnalyticalQualityCommission,Spaine
InstitutoNacionaldeSaude,Portugalf
BloodSciences,OldMedicalSchool,LeedsTeachingHospitalsTrust,Leeds,UKg
DepartmentofClinicalChemistry,LeidenUniversityMedicalCenter,Leiden,TheNetherlandsh
DepartmentofClinicalChemistryandHematology,AmphiaHospital,Breda,TheNetherlands
b
articleinfoabstract
Introduction:Inthemodernhealthcareservice,patientsreceivecareinmultiplehospitalsandhealthcaresettings.Therefore,harmonizationofresultsfromdifferentmethodsandinstruments,bothbetweenandwithinlaborato-ries,isoftheutmostimportance.ThepresentpilotstudyaimstotesttheuseofaCategory1EQAschemeacrossfourEuropeancountriesbyassessingthecurrentlevelofequivalenceoftestresults.
Method:ThisworkwasledbytheDutchExternalQualityAssuranceSchemeSKMLandinvolved28laboratoriesfromthreeregionsintheUK,SpainandPortugal,and120laboratoriesfromTheNetherlands.Asetofsixcom-mutablesamples,targetedwithreferencemethods,werecirculatedand18biochemistryanalytesweretested.Resultsandconclusions:TheTotalError(TE)score,de?nedastheprobability(%)thatresultsarewithintheTotalErrorAcceptable(TEA)limits,fortheeighteenanalyteswascalculated.Ourdatashowthatthereisaneedforfurtherharmonizationoflaboratorydata,inparticularforelectrolytes(calcium,chloride,magnesium,sodium),enzymes(ALT,amylase,AST,LDH),lipids(HDL-cholesterol),andforsubstrates(creatinine,totalprotein).Lackofperformanceconsistencybetweeninstrumentswasseenformostanalytes.Thelackofharmonizationisstillpresentdespitemanufacturerclaimsofestablishedtraceability.
?2013ElsevierB.V.Allrightsreserved.
Articlehistory:
Received25May2013
Receivedinrevisedform5November2013Accepted5November2013AvailableonlinexxxxKeywords:
ExternalqualityassessmentCommutabilityBiologicalvariationReferencemethodTraceabilityHarmonization
1.Introduction
Mosteffortsinthemanagementofanalyticalqualityinclinicalchemistryandlaboratorymedicinehavefocusedonthereductionofwithin-laboratoryvariationandtheassessmentofbetween-laboratoryvariation.Inrecentyearstheimportanceofminimizingbias,bothbetweenlaboratoriesandwithinalaboratory,hasbecomeparamount.Patientsarefrequentlytreatedbyateamofphysiciansratherthanone,oftenextendingacrossseveralhealthcaresettingsandmakinguseofinformationfromseverallaboratories.Inmonitoringpatientsduringtreatment,theabsenceofbiasfromonemeasurementtothenext,togetherwithminimumimprecisionisessential.Calibrationandharmonizationofresultsfromdifferentanalyzers,bothbetweenand
Abbreviations:EQA,externalqualityassessment;TE,totalerror;IVDD,invitrodiagnosticmedicaldevicesdirective.?Correspondingauthor.
E-mailaddress:of?ce@skml.nl(R.Jansen).


0009-8981/$–seefrontmatter?2013ElsevierB.V.Allrightsreserved./10.1016/j.cca.2013.11.003
withinlaboratories,andthecontinuityofsuchharmonizationintimeare,therefore,oftheutmostimportance.Smallassaybiasesmayhavealargeimpactonpatientclassi?cationandonthenumberofpatientstobetreated,particularlyforassaysforwhichcut-offvaluesareused.Thisistrue,forexample,inlipidandlipoproteinanalyses,inwhichstringentcut-offvaluesareusedthroughouttheworldforthepreven-tionandtreatmentofcardiovasculardiseases.Itisalsotrueforcreati-nineintheestimationofrenalfunctionandforhumangrowthhormoneinhGHde?ciency.
TheAmericanAssociationforClinicalChemistry(AACC)conferenceinOctober2010focusedontheroadmap[1]toreachharmonizationforanalytesforwhichnoreferencesystemisde?ned.However,evenforanalytesforwhichsuchsystemsexist,standardizationisoftenlacking.Theprocessisde?nedasstandardizationiftheanalyteisclearlyde?nedandreferencemethodandstandardsexist.Harmonizationiscon?nedtodescribeprocesseswhereoneormoreoftheseelementsaremissing.ExternalQualityAssessment(EQA)schemesshouldplayacentralroleinachievingharmonizationandintruenessveri?cation.Itiswidely
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
2R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx
Creatinine
30252015Difference μmol/L
1050-5-10-15-20-2560
80
100
120
140160μmol/l
180
200
μmol/l
SampleYouA120B191C154D146E207F78Total149Precision1.8%Count6Outliers0
Ref126.3196.0157.0149.0219.478.7154
Cons12619xxxxxxxxxxxx5675442
CV5.1%3.6%5.2%3.8%3.3%9.1%4.2%2.7%
Meth1271961621622188215626114
Anal12619213914921580151602
220240
Regression line Y=7 + 0.920XStatistics valid for your
Method:Alk. Picrate, kinetic with compensation-group:Jaffe
Analyser:Olympus AU2700
Fig.1.ExampleofadifferenceplotoftheCombischeme.ThegreenareaisTEAtoleranceareaaroundthereferencemethodtarget.Theblueareaisstateofthearttolerancelimitaroundtheconsensusmeanvalue.ThegreensquaresareyourresultsforsamplesA–F.Theblacklineisyourregressionline.Verticalbarsindicate±3SDbetweenlabs.Precisioniswithin-labCV.Anal.isyouranalyzer.
acceptedthatweneedcommutablematerials[2,3],referencemethodtargetvaluesandtolerancelimitsbasedonthebiologicalvariationconcept[4–6].EQAschemeshavingthesecharacteristicshavebeendenotedasCategory1schemes[7].TheimportanceofusingthisconceptinEQAschemeswasstressedrecentlyinseveralsessionsduringtheBio-RadConvocationofExpertsonLaboratoryQuality2010inBardolino,Italy[8]againin2011inSalzburg,Austria.IntheCalibration2000projectoftheDutchNEQASorganizerSKML,thiswasachievedforseveralanalytes[9–15].TheInVitroDiagnosticMedicalDevicesDirective(IVDD)requirestraceabilitytoreferencesystems[16].Thesesystemsarede?nedforanumberofanalytes.Fortheseanalytestruenessveri?cationispossibleandharmonizationiswithinreach.
TheCalibration2000projectinTheNetherlandsproducesmaterials[9–15]forgeneralclinicalchemistry,proventobecommutableinconformitywiththeClinicalandLaboratoryStandardsInstitute(CLSI)C53A[17].Thesamplesaretargetedwithreferencemethods,undertakenineitherTheJointCommitteeforTraceabilityinLaboratoryMedicine(JCTLM)listedReferencelaboratoriesorinInternationalFederationofClinicalChemistryandLaboratorymedicine(IFCC)networklaboratories,ifavailable,andresultsareprocessedintheCombiEQAscheme[11–14]inwhichparticipatinglaboratoriesassayseveralsamplescoveringtheclinicallyrelevantconcentrationrange.TheschemeusesthebiologicalvariationbasedTotalErrorallowable(TEA)atthedesirablelevelastolerancelimit.Harmoniza-tionofminimalacceptableperformancecriteriaamongEQAorga-nizersisdesirable[18].
Thepresentstudyisapilotstudy.ItaimstotesttheuseofaCategory1EQAschemeacrossthecountries,UK,Spain,PortugalandTheNetherlands,andtocompareinapilotstudytheperformanceoftheparticipatinglaboratoriesandthemethodsused.Theresultsofthepilotstudyareseenasapreliminaryviewoftheroleofcategory1EQAtoimproveharmonizationinEurope.2.Methods
IntheSKMLCombischeme,24samplesareanalyzedforgeneralchemistryparametersinthecourseofayear,i.e.atafrequencyofonesampleper2weeks.Forlipids,aseparatededicatedbatchof24samplesisused.Thesamplesarepreparedaccordingtoexactlythesameproto-colaspreviouslypreparedsampleswhichwereproventobecommut-able[9–15].Inshort,twomastersamplesareprepared,onefrompoolednormalhumanleftoverseraandonefrompoolednormalhumansera,spikedwithabnormalpools,minerals,recombinanthumanenzymesandhumanalbumin.Themasterpoolsaremixedintenproportionsthusobtaining12concentrationlevels.Afterdispens-ing,vialsarefrozenat?84°C.Previouslypreparedsamplesaccordingtothisprocedurewererepeatedlyproventobecommutable,whethermaster,spikedormixedsamples.Throughouttheyearscommutabilityhasbeenmonitoredbyincludinganative,singledonationspy-samplethatispreparedaccordingtoCLSIC37-A2.Concentrationscovertherangeofclinicalinterest.ThesamplesaretargetedbyJCTLMlistedlaboratoriesforelectrolytesandsubstrates,andbyIFCCorCDCnetworklaboratoriesforenzymesandlipids.Informationonrefer-encemethodsandlaboratoriesusedisprovidedassupplementarydata.Biologicalvariationbasedtolerancelimitsareused(TEAdesirable).
ThirtylaboratoriesfromthreeEuropeancountriesparticipatedinthisstudyinadditionto120regularlyparticipatingDutchSKMLEQACombischeme.TenlaboratorieseachparticipatedfromtheUK,Spain(onelabwithtwoproceduresforallanalytes,exceptforlipids)andPortugal.TheUKlaboratories'inclusioninthisstudywassolelybasedonexpressionofinterestfromlaboratoriesthathadreceivedaninvitationtoparticipate.Theauthorshavehadnopreviousknowledgeoftheanalyticalperformancefortheparticipatinglaboratories.
TheSpanishandthePortugueselaboratorieswereselectedfromthoselaboratoriesfallingwithinthe20thpercentileofthetargetdevia-tionoftheirnationalEQAschemes.However,theparticipatinglaborato-riesrangefromsmallindependenthealthcarelaboratoriestolargelaboratoriesservingteachinghospitals,amixofsizeandanalyticalplat-forms,whichre?ectsthesamedistributionineachcountry.
Asetofsixsamplesforgeneralchemistryandasetofsixsamplesforlipidsfrozenat?80°C,weretransportedondryicetoacentrallabora-toryineachofthethreecountries,andstoredat?80°C.Thefrozensamplesweredistributedondryicefromthecentrallaboratorytotheparticipatinglaboratories.SamplesarrivedthawedintwoPortugueselaboratoriesandthesewerediscarded.Sincemostofthelaboratories
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx3
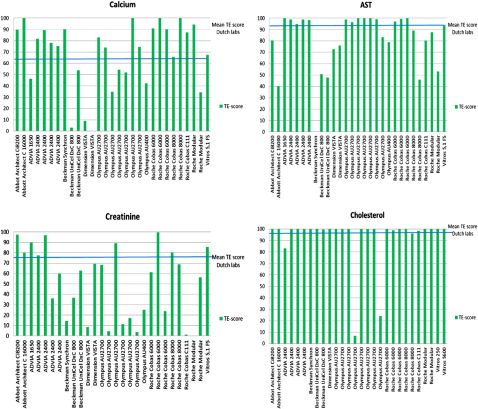
Fig.2.TEscoresperanalyticalplatformfor28laboratories.TEscoresvaryconsiderablywithinusersofinstrumentsfromthesamemanufacturer.ThebluelinerepresentstheaverageTEscoreforTheNetherlandslaboratories.
didnothavea?80°Cfreezeravailable,thelaboratorieswereaskedtoanalyzethesamplesassoonaspossibleafterreceiptortostorethesam-plesat?20°Candanalyzethemwithin1week(theperiodofstability,asdeterminedbythesampleprovider).TheDutchparticipantsreceivedtheirsetsofsamplesatthestartoftheyearandkeptthemat?80°Cuntilanalysis.
Laboratorieswereaskedtoanalyze18analytesforwhichtargetvalueswereobtainedfromreferencelaboratoriesusinginternationallyrecognizedreferencemethodsandreferencematerials.Theycomprised:
5electrolytes:calcium,chloride,magnesium,potassium,sodium;6enzymes:ALT,amylase,AST,CK,Gamma-GT,LDH;2lipids:cholesterol,HDL-cholesterol;and
4substratesandaformula:creatinine,eGFR(F,55y,Caucasian),glucose,totalprotein,uricacid.
Thelaboratorieswereaskedtousetheirroutinemethodswithnoadaptationscomparedtoroutinepractice.Thelaboratoriesreportedtheirresults,methods,andtheinstrumentsused.ThelaboratoriesintheUKandSpainmostlyreportedSIunits,whilethelaboratoriesinPortugalmostlyreportedconventionalunits.Conventionalunitswere
convertedtoSIunitsbytheorganizerofthepilotstudy.Inafewcasesthereportedresultswerenotinagreementwiththereportedunitsandcorrectionsweremade.OneSpanishlaboratoryreportedcreatinineinSIunitsafterconvertingfromconventionalunits(mg/dL),butusingawrongconvertingfactor.ThismistakedidnotaffecttheeGFRresults,becauseaformulaforcreatininevaluesinmg/dLwasused.Resultsforcreatinineofthislaboratorywerediscarded.FourSpanishlaboratoriesusedapancreaticamylaseassayinsteadoftotalamylaseandtheirre-sultswerediscarded.2.1.Statisticalmethods
IntheCombischemereport,eachroundandforeachanalytetheindividuallaboratorydataisdisplayedasadifferenceplotofthesixresultscomparedwiththereferencemethodvalues(Fig.1).AtoleranceareaisconstructedaroundthereferencevaluesbasedontheTEA(desirable)limit[4,5].IntheCombischemethedesirableTEAlimit(TEA=1.65×0.5×CVw+0.25√(CVw2+CVb2))isusedratherthantheminimaloroptimallimitsasalternativeapproachessuggestedbyFraser[6].Linearregressioniscalculatedfromthelaboratoryresultsagainsttheconsensusmethodgroupmeanvalue.Asthesamples
are
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
4
Table1
TEA,averageTEscores,and%TEscores≥95%.Analyte
TEA%
CalciumChlorideMagnesiumPotassiumSodiumALT
AmylaseASTCK
Gamma-GTLDH
Cholesterol
HDL-cholesterolCreatinine
eGFR(F,55y,Caucasian)Glucose
TotalproteinUricacidOverall
2.41.54.85.80.914.626.315.230.322.211.48.511.18.98.97.23.412.4
NLAvTEscore(%)64646194479385949997849783766693589881
R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx
NL%TEscN95%18162877584778296937687554147672896
PTAvTEscore(%)653957892680537683xxxxxxxxxxxx88539367
PT%TEscN95%001363063433863xxxxxxxxxxxx631363
ESAvTEscore(%)64816797428359889890639074336492779975
ES%TEscN95%2730228294540649191559060027733691
UKAvTEscore(%)7372799747xxxxxxxxxxxx882655796649977
UK%TEscN95%1030307020xxxxxxxxxxxx06020259030100
measuredondifferentdays,theresidualSDoftheregressionlinerepre-sentsthewithin-laboratorySDWL.Thedifferencebetweenthemeanofthesixlaboratoryresultsandtheaverageofthesixreferencevaluesisthebias.UsingSDWLattheaverageconcentrationofthesixsamplestheprobabilityisestimatedthatthelaboratoryresultswillbewithintheTEAtolerancearea.Thisprobabilityisthepercentageofthedensityfunction(thebroadnessofwhichisde?nedbySDWL)aroundthelaboratorybiasthatiswithintheTEAarea.TheTEscoreequalsthispercentage.Byde?nitionTEincludesbiasandimprecision.CausesoflowerTEscorescouldbesigni?cantpositiveornegativebias,oralargewithin-laboratorySD.Increasingly,minimalacceptableperformancecriteriabasedonthebiologicalTEAconceptarebeingutilizedwithinlaboratories.Thelevelofacceptanceisde?nedbyFraser[6]asminimal,desirableoroptimal.IntheSKMLscheme,performanceisconsideredtobeacceptableiftheresultsofalaboratoryarewithinthedesirableTEAareawithaprobabilityof95%.
ForeachanalytetheTEscoresoftheindividuallaboratoriesoftheUK,SpainandPortugalwereplottedagainstandcomparedwiththeaverageTEscoreoftheDutchlaboratories.Foreachanalyte,theindivid-uallaboratoryresultssortedbyinstrumentwerealsoplotted.AverageTE-scoresandthepercentageTEscoresN95%werecalculatedforthefourcountries.3.Results
Fig.1showsanexampleofadifferenceplotofthesixresultsofasinglelaboratoryforcreatinine.ThegreenarearepresentstheTEAareaaroundthereferencevalues.Theblueareaisthestateofthearttoleranceareaforthemethodgroupconsensusvalues.TheblueareainthiscaseshowsapositivedeviationfromthereferencevaluesinthelowerconcentrationrangeasisexpectedfortheJaffemethodgroup.TheplotshowsyourregressionversustheTEAtolerancearea,versusyourmethodgroupstateofthearttolerancearea,andthemethodgroupstateoftheart(bluearea)versustheTEAgreenarea.Thewithin-labCViscalculatedastheresidualCVoftheregressionline.TheTEscoreforthislaboratoryequals97%.IntheSKMLCombicon-ceptaTEscoreof95%isconsideredacceptable.Nexttothedifferenceplotatableisreportedtotheparticipantssummarizingtheresults.
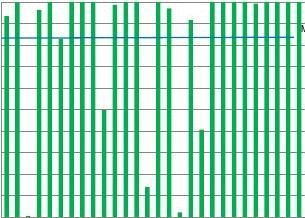
Thereferencevaluesofthesixgeneralchemistryandthesixlipidsamplesfortheselected18analytes,arrangedbyanalytegroup(electrolytes,enzymes,lipids,substrates),thestandarduncertainties,thereferencemethodsandthereferencelaboratories,areprovidedassupplementarydata.Fig.2showsanexampleofTEscoresofindividuallaboratoriesforfouranalytessortedbyinstrument.Withtheexceptionofcholesterol,theotherthreeanalytes(AST,calciumandcreatinine)showinconsis-tencyofTEscoresevenwithinasingleanalyticalplatform.Thislackofperformanceconsistencybetweeninstrumentsandwithininstrumentwasseenforallelectrolytes,andenzymes(datanotshown),andisinagreementwithpreviouslyreportedresults[19].
Table1presentstheTEAvalues[5],averageTEscoresofthefourcountriesandthepercentageoflaboratoriesthathadaTEscore≥95%.TheNetherlands'TEscorewasthehighestat81%,followedbytheUK'sat77%,Spain'sat75%andPortugal'sat67%.
TEscoresforallelectrolytes,exceptpotassium,inallofthefourcountriesarelow(Table1,Fig.3).Urgentimprovementinharmoniza-tionisneededparticularlyforcalcium,chloride,magnesiumandsodiumwherelessthan30%ofthetrueTEscoreswereabovethe95%criterion.Thesameobservationwasmaintainedfortheenzymes.ThehighestTEscorewasseenforCKandGGT.However,awidevariationinTEscorewithinandbetweencountrieshasbeenrecordedforALT,ASTandamylase.Foramylase,laboratoriesshowtwotypesofTEscores,eitherTEabove95%oraverylowscoreoftenzero.CalibrationtoamethoddifferentfromthereferencemethodisthemaincauseoflowTEscores.FourSpanishlaboratoriesanalyzedpancreaticamylaseinsteadoftotalamylase.Theresultsoftheselaboratorieswereremovedastheyweretestingadifferentanalyte.
WiththeexceptionofTheNetherlands,Portugal,SpainandtheUKshowedpoorTEscoresforLDHwithmanyscoresofzeroobtainedand19outof28laboratorieshavingscoresbelow10%(Fig.4).ThisisduetothefactthatanumberoflaboratoriesareusingapyruvatetolactatemethodratherthantheIFCCreferencemethodutilizinglactateasasubstrate.Thesemethodsvarybyafactorof2andwillthereforehaveaprofoundeffectonbias,whichexplainsthepoorTEscorefortheselaboratories.
IngeneraltheTEscoresforenzymesinTheNetherlandsarehigher,andalargerpercentageofthelaboratoriesscoreabovethe95%limit,ascomparedtotheothercountries.
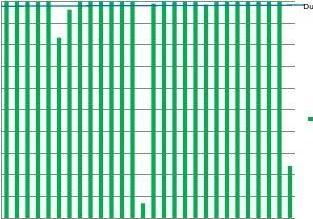
ForcholesteroltheaverageTEscoreswereabove90%andover85%ofthelaboratoriessatis?edthecriterionofTEscore≥95%(Table1andFig.5).However,theHDLmethodshavenotmatchedtheconsis-tentlyhighperformanceseenwithcholesterol.WhilethePortugueseachievedaTEscoreof100%foralltheparticipatinglaboratories,othercountriesdemonstratedawidervariationinperformance(Fig.5).
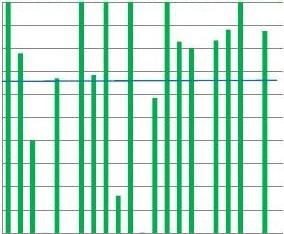
Forcreatinine(Table1andFig.6)lowaveragescoreswereobtainedaswellaslowpercentagesofTEscores≥95%,indicatingthatmany
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx5
1009080706050
Calcium
100908070
Mean TE scoreDutch labs
Chloride
6050
Mean TE scoreDutch labs
TE-score
TE-score
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT080
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT080
Magnesium
1009080706050
TE-scoreMean TE scoreDutch labs
Potassium
1009080706050
TE-scoreMean TE scoreDutch labs
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT080
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT080
Sodium
10xxxxxxxxxxxx302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT080
TE-scoreMean TE scoreDutch labs
Fig.3.Electrolytes,TEscoresofindividuallaboratoriespercountry(region).ThebluelinerepresentstheaverageTEscoreforTheNetherlandslaboratories.
laboratoriesfailedtoachieveminimalacceptableperformance.Instru-mentsshowedwidelyvaryingresults.TheJaffémethodshadthelowestscore(datanotshown),whichisinagreementwithpreviouslyreportedresults[20].
ThishasaconsequenceforeGFR,whichwascalculatedusingdifferentformulaethe23participatinglaboratories.AverageTEscoreswerebelow70%andlessthanhalfofthelaboratoriesattainedaTEscoreof95%.GlucoseanduricacidmettheacceptableperformancecriterionofaTEscoreN95%forthemajorityofparticipatinglaboratoriesinthefourcountries.
ThedataforTotalProteinindicatedunsatisfactoryperformance,withaverageTEscoreswellbelow95%andmorethan70%ofthelaboratoriesfailingthe95%criterion.Fig.6illustratesthewidelyvaryingindividualscores.
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
6R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx
ALT
1009080706050
TE scoreMean TE score Dutch labs
AST
TE scoreDutch labs
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT080
UK0UK0UK0UK0UK0UK0UK0UK0UK0UK1ES0ES0ES0ES0ES0ES0ES0ES0ES0ES0ES1PT0PT0PT0PT0PT0PT0PT0PT0Amylase
UK0UK0UK0UK0UK0UK0UK0UK0UK0UK1ES0ES0ES0ES0ES0ES0ES1PT0PT0PT0PT0PT0PT0PT0CK
10090
Dutch labs
80706050
TE-score
TE-score
40302010
UK0UK0UK0UK0UK0UK0UK0UK0UK0UK1ES0ES0ES0ES0ES0ES0ES0ES0ES0ES0ES1PT0PT0PT0PT0PT0PT0PT0PT00
Gamma-GT
UK0UK0UK0UK0UK0UK0UK0UK0UK0UK1ES0ES0ES0ES0ES0ES0ES0ES0ES0ES0ES1PT0PT0PT0PT0PT0PT0PT0PT0TE-scoreDutch labs
LDH
1009080706050
TE-scoreMean TE score Dutch labs
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT080
Fig.4.EnzymeTEscoresofindividuallaboratoriespercountry(region).ThebluelinerepresentstheaverageTEscoreforTheNetherlandslaboratories.
4.Discussion
IntheEuropeanUnion,theIVDD98/79/EC[21]demandstraceabilityoftestresultstoahigherorderreferencematerial.Thismeansthattheresultsforeachinstrumenttypeshouldbecomparablewithreferencemethodresults.However,thispilotstudyshowsconsiderablewithininstrumentandbetweenlaboratoryvariationsinTEscores.AlthoughthenumberofparticipatinglaboratoriesfromoutsideTheNetherlandsissmall,theymaybeconsideredasrepresentativeofcountriesbecausetheyarepositionedwithinthe20thpercentileofthetargetdeviationdistributionintheirnationalEQAschemes(Spain,Portugal)orarerepresentativeforawholeregion(Yorkshire,UK).
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx7
Cholesterol
UK0UK0UK0UK0UK0UK0UK0UK0UK0UK1ES0ES0ES0ES0ES0ES0ES0ES0ES0ES1PT0PT0PT0PT0PT0PT0PT0PT0
HDL-Cholesterol
Dutch labs
Reeks2
UK0UK0UK0UK0UK0UK0UK0UK0UK0UK1ES0ES0ES0ES0ES0ES0ES0ES0ES0ES1PT0PT0PT0PT0PT0PT0PT0PT0Fig.5.LipidTEscoresofindividuallaboratoriespercountry(region).ThebluelinerepresentstheaverageTEscoreforTheNetherlandslaboratories.
Jansenetal.showedin2006[19]thatlargevariationbetweenmethodsandanalyticalplatformsexistfortheenzymesandthatinmanycasesthereisalackoftraceabilityandharmonizationdespitetheIVDrequirements.Thisstudyshowslittleimprovementwithen-zymeassays,especiallyforamylase[22]andLDH[22,23].LaboratoriesarestillusingmethodsthatdonotcomplywiththeIFCCrecommendedmethods(e.g.triosidesubstrateforamylaseorpyruvatesubstrateforLDH)andthisshouldbediscouraged.Furthermore,insomeenzymemethodse.g.ALT/AST,variationinTEscorehasbeenseenwithintheusersofthesameinstrument.Inourview,this?ndingmaybeattributedtotheuseofALT/ASTmethodslackingtheadditionoftheco-enzymepyridoxalphosphateinthereagentpack.Theco-enzymehasavariableandmarkedeffectontransaminaseactivity,especiallywithAST,whichcannotbecorrectedbycalibration.DespitetheIFCCrecommendations[24,25],manufacturersstillmarketmethodversionslackingpyridoxalphosphate.Methodsthatdonotcontaintheco-enzymecannotbecon-sideredtraceable.Anothersourceofdiscrepant(biased)resultshasbeenobservedforaSpanishlaboratory(datanotshown)forASTandgamma-GTwhenaroutinecalibratorwastraceabletoanon-commutablereferencematerial,whereasresultswerecorrectwhencor-rectlycalibratedandtraceabletoareferencemethod.Intheseexamples,themanufacturerscanplayapivotalroleinpavingtheroadtoharmoni-zation,simplybyremovingundesirablemethodsfromthemarket.
ThevariationintheTEscoresinUK,SpainandPortugalcannotbeexplainedbythedifferentanalyticalplatforms.Fig.2showsexamplesofTErangesfortheinstrumentsused.WithinthesameinstrumentTEscoresvarygreatly,insomecasesfrom0%to100%.Oneexplanationforthisistheproductionbymanufacturersofmorethanoneassayonthesameplatformforsomeanalytes,e.g.LDHlactatetopyruvateandpyruvatetolactate,andAST/ALTwithandwithoutpyridoxalphosphateP5P,whilsttraceabilitydemandstheIFCCrecommendations.Otherrea-sonscouldbebiasduetotheuseofdifferentfactors,differentcalibra-tors,andvaryingwithin-laboratorySD.Thebiascouldbeproportional,constantormixedi.e.varyingacrosstheconcentrationspan.Inspectionofthedatashowsthatinmanycasesalloftheseerrorsarepresent.E.g.forcreatininemanylaboratoriesusethenon-compensatedJaffékineticmethod,givingapositivebiasatlowconcentrationlevel.LaboratoriesneedtoshowacceptableprecisionaswellasbiastoattainaTEscoreof95%.LackofcommutabilityofthereferencematerialusedforroutinecalibratortraceabilityhasbeenseenasamajorreasonforbiasedresultsintheSpanishgroup.Thesamehappensformagnesiumandsodium.Ourdatashowsthaturgentimprovementinharmonizationisneededparticularlyforcalcium,chloride,magnesiumandsodiumwhereless
than30%oftheTEscoreswereabovethe95%criterion.Harmonizationofanalytesthathaveanarrowbiologicalvariabilitycanbeimprovedbysharingacommonbutclinicallyrelevantanalyticalgoal[26].Examplesofdifferentkindsoferrorsmadeareprovidedassupplementarydata.
Alltheanalytesinthisstudyhaveawell-de?nedreferencemeasure-mentprocedureandtraceabilitychain,yetconsiderableanalyticalvariationhasbeenseen.Thissuggeststhatstandardizationaloneisnotsuf?cienttoguaranteeproductionofcomparableresults.Traceabil-ityofamethodtohigherorderreferencemeasurementmethodsdoesnotnecessarilymeanthatthe?eldmethodresultsareidenticaltothereferencemethodresults.Itrequiresafunctionalrelationshipbetweenthemethodandthereferencemethodandreferencematerial.Fromapatient'sperspective,resultsfromdifferentlaboratoriesshouldnotonlybetraceabletothereferencemethod,i.e.showade?nedfunctionalrelationshiptothereferencemethod,butshouldinadditionbestan-dardized,i.e.giveequivalentresultstothereferencemethod.TheCali-bration2000project[9–11,15]andthepresentresultsshowthatharmonizationisachievableforsomeanalytesasshownintheCategory1EQAscheme.TheCombischemeinitspresentform,usingcommut-ablesamples,valueassignedwithreferencemethods,andhavingbiologicalvariation-basedtolerancelimits,hasbeenoperationalinTheNetherlandsforover7years.InanattempttoreplicateTheNetherlandsexperiencewithlargernumbersoflaboratories,thePortuguese,theSpanishandtheUKEQAschemeorganizersarecon-sideringcollaborationinatleastoneroundperyearintheSKMLCombischeme.
Since2005,theSpanishSocietyofClinicalChemistry(SEQC)haveundertakenaneducationaltaskinrecommendingtheuseofbiologicalvariationbasedastolerancelimitsandthesecriteriaareincludedintheparticipants'reports.Despitethis,thegroup'sresultsarenotassatisfactoryastheyshouldbe.Thisismainlyduetothelackofmethodharmonizationandtraceabilityandnottoadifferentcultureinqualitymonitoringpractices.5.Conclusion
TheIVDD98/79/ECdemandstraceabilityoftestresultstoareferencesystem,ifavailable.Ourdatashowthatthereisaneedforfurtherharmonizationoflaboratorydata,inparticularforelectrolytes(calcium,chloridemagnesium,sodium),enzymes(ALT,amylase,AST,LD),lipids(HDL-cholesterol),andforsubstrates(creatinine,totalprotein).Lackofperformanceconsistencybetweeninstrumentswasseenformostanalytes.ThevariationintheTEscorescannotbeexplainedbythe
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
8R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx
1009080706050
Creatinine
10090
Dutch labs
80706050
Dutch labs
TE-score
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT08

403020100
UK01UK02UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT05
Dutch labs
TE-score
1009080706050
Glucose
1009080706050
Total Protein
Dutch labs
TE-score
TE-score
403020100
403020100
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT08
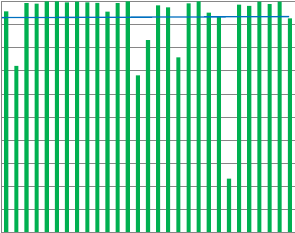
1009080706050
Urate
Dutch labs
TE-score
40302010
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT08
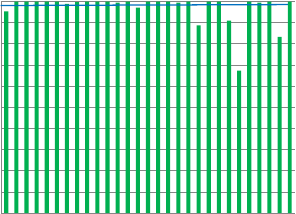
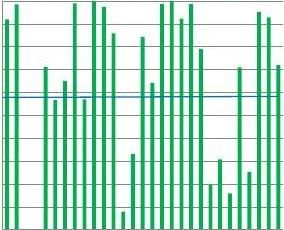
Fig.6.SubstrateTEscoresofindividuallaboratoriespercountry(region).ThebluelinerepresentstheaverageTEscoreforTheNetherlandslaboratories.
differentanalyticalplatforms.WithinthesameinstrumentTEscoresvarygreatly,insomecasesfrom0%to100%.Lackofharmonizationisstillpresent,despitemanufacturers'claimsofestablishedtraceability.Currentdatashowsthatthestandardizationofmethodsisinsuf?cienttoresultincompleteconsistencyinreportingoflaboratoryresultsandneedstobefollowedbyharmonizationofthemethodsandpractices.
Declarations
Competinginterests:noneFunding:none
Ethicalapproval:notrequiredGuarantor:DrRobJansen
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
UK01UK02UK03UK04UK05UK06UK07UK08UK09UK10ES01ES02ES03ES04ES04ES05ES06ES07ES08ES09ES10PT01PT02PT03PT04PT05PT06PT07PT08
R.Jansenetal./ClinicaChimicaActaxxx(2013)xxx–xxx9
Acknowledgments
Thecontributionofthe30laboratoriesinPortugal,SpainandtheUKinperformingtheanalysisofthesixsamplesandinthesubmissionoftheresultsarekindlyacknowledged.AppendixA.Supplementarydata
[16][14]
[15]
Supplementarydatatothisarticlecanbefoundonlineat/10.1016/j.cca.2013.11.003.References
[1]MillerWG,MyersGL,GantzerML,KahnSE,Sch?nbrunnerER,ThienpontLM,etal.
Roadmapforharmonizationofclinicallaboratorymeasurementprocedures.ClinChem2011;57:1108–17.
[2]ThienpontLM,St?cklD,FriedeckyB,KratochvílaJ,BudinaM.Truenessveri?cationin
Europeanexternalqualityassessmentschemes:timetocareaboutthequalityofthesamples.ScandJClinLabInvest2003;63:195–201.
[3]MillerWG,MyersGL,RejR.Whycommutabilitymatters.ClinChem2006;52:553–4.[4]FraserCG.Generalstrategiestosetqualityspeci?cationsforreliabilityperformance
characteristics.ScandJClinLabInvest1999;59:487–90.
[5]MinchinelaJ,RicósC,PerichC,Fernández-CalleP,AlvarezV,DomenechM,etal.Bi-ologicalvariationdatabase,andqualityspeci?cationsforimprecision,biasandtotalerror(desirableandminimum).The2012update/biodatabase-2012-update.htm.[accessed08-07-2013].
[6]FraserCG,HyltoftPetersenP,LibeerJC,RicósC.Proposalsforsettinggenerally
applicablequalitygoalssolelybasedonbiology.AnnClinBiochem1997;34:8–12.[7]MillerGW,JonesGRD,HorowitzGL,WeykampC.Pro?ciencytesting/externalquality
assessment:currentchallengesandfuturedirections.ClinChem2011;57:1670–80.[8]AdamsO,CooperG,FraserC,HubmannM,JonesG,PlebaniM,etal.Collective
opinionpaperon?ndingsofthe2011convocationofexpertsonlaboratoryquality.ClinChemLabMed2012;50:1547–58.
[9]JansenRTP.Thequestforcomparability:Calibration2000.AccredQualAssur
2000;5:363–6.
[10]JansenRTP,KuypersAWHM,BaadenhuijsenH,BesselaarAMHPvanden,Cobbaert
CM,GratamaJW,etal.Kalibratie2000.NedTijdschrKlinChem2000;25:153–8.[11]BaadenhuijsenH,ScholtenR,WillemsHL,WeykampCW,JansenRTP.Amodelfor
harmonizationofroutineclinicalchemistryresultsbetweenclinicallaboratories.AnnClinBiochem2000;37:330–7.
[12]BaadenhuijsenH,SteigstraH,CobbaertC,KuypersA,WeykampC,JansenR.
Commutabilityassessmentofpotentialreferencematerialsusingamulticentersplit-patient-samplebetween-?eld-methods(twin-study)design:studywithintheframe-workoftheDutchproject“Calibration2000”.ClinChem2002;48:1520–5.
[13]CobbaertCM,WeykampC,BaadenhuijsenH,KuypersA,LindemansJ,JansenR.
Selection,preparation,andcharacterizationofcommutablefrozenhumanserumpoolsaspotentialsecondaryreferencematerialsforlipidandapolipoprotein
[17]
[18]
[19]
[20]
[21][22]
[23]
[24]
[25]
[26]
measurements:studywithintheframeworkoftheDutchProject“Calibration2000”.ClinChem2002;48:1526–38.
BaadenhuijsenH,KuypersA,WeykampC,CobbaertCM,JansenR.ExternalqualityassessmentinTheNetherlands:timetointroducecommutablesurveyspecimens.LessonsfromtheDutch“Calibration2000”project.ClinChemLabMed2005;43:304–7.
CobbaertCM,WeykampC,FranckP,deJongeR,KuypersA,SteigstraH,etal.Sys-tematicmonitoringofstandardizationandharmonizationstatuswithcommutableEQA-samples—?veyearexperiencefromTheNetherlands.ClinChimActa2012;414:234–40.
MüllerMM.Implementationofreferencesystemsinlaboratorymedicine.ClinChem2000;46:1907–9.
ClinicalandLaboratoryStandardsInstitute(CLSI).Characterizationandquali?cationofcommutablereferencematerialsforlaboratorymedicine;approvedguideline.CLSIC53-A940WestValleyRoad,Suite1400,Wayne,Pennsylvania19087-1898USA:ClinicalandLaboratoryStandardsInstitute1-56238-726-X;2010.
RicósC,RamónF,SalasA,BunoA,CalafellR,MoranchoJ,etal.Minimumanalyticalqualityspeci?cationsofinter-laboratorycomparisons:agreementamongSpanishEQAPorganizers.ClinChemLabMed2012;50(3):455–61.
JansenR,SchumannG,BaadenhuijsenH,FranckP,FranziniC,KruseR,etal.Truenessveri?cationandtraceabilityassessmentofresultsfromcommercialsystemsformeasurementofsixenzymeactivitiesinserum.AninternationalstudyintheEC4frameworkoftheCalibration2000project.ClinChimActa2006;368:160–7.
DelangheJR,CobbaertC,HarmoinenA,JansenR,LaitinenP,PanteghiniM.Focusingontheclinicalimpactofstandardizationofcreatininemeasurements:areportbytheEFCCWorkingGrouponCreatinineStandardization.ClinChemLabMed2011;49:977–82.
LexEU.Directive98/79EConinvitromedicaldevices.OffJL1998;331:1–37.
SchumannG,CanaliasF,JoergensenPJ,KangD,LessingerJM,KlaukeR.IFCCreferenceproceduresformeasurementofthecatalyticconcentrationsofenzymes:corrigendum,notesandusefuladvice.InternationalFederationofClinicalChemistryandLaboratoryMedicine(IFCC)—IFCCScienti?cDivision.ClinChemLabMed2010;48:615–21.
SchumannG,BonoraR,CeriottiF,Clerc-RenaudP,FerreroCA,FérardG,etal.IFCCprimaryreferenceproceduresforthemeasurementofcatalyticactivityconcentra-tionsofenzymesat37°C:Part3.Referenceprocedureforthemeasurementofcat-alyticconcentrationoflactatedehydrogenase.ClinChemLabMed2002;40:643–8.SchumannG,BonoraR,CeriottiF,FérardG,FerreroCA,FranckPF,etal.InternationalFederationofClinicalChemistryandLaboratoryMedicine.IFCCprimaryreferenceproceduresforthemeasurementofcatalyticactivityconcentrationsofenzymesat37°C.InternationalFederationofClinicalChemistryandLaboratoryMedicine:Part4.Referenceprocedureforthemeasurementofcatalyticconcentrationofalanineaminotransferase.ClinChemLabMed2002;40:718–24.
SchumannG,BonoraR,CeriottiF,FérardG,FerreroCA,FranckPF,etal.InternationalFederationofClinicalChemistryandLaboratoryMedicine.IFCCprimaryreferenceproceduresforthemeasurementofcatalyticactivityconcentrationsofenzymesat37°C.InternationalFederationofClinicalChemistryandLaboratoryMedicine:Part5.Referenceprocedureforthemeasurementofcatalyticconcentrationofaspartateaminotransferase.ClinChemLabMed2002;40:725–33.
JassamN,LindsayC,HarrisonK,ThompsonD,BosomworthMP,BarthJH.Theimple-mentationofasystemformanaginganalyticalqualityinnetworkedlaboratories.AnnClinBiochem2011;48:136–46.
Pleasecitethisarticleas:JansenR,etal,Acategory1EQAschemeforcomparisonoflaboratoryperformanceandmethodperformance:Aninter-nationalpilotstudyintheframeworkoftheCalibration2000project,ClinChimActa(2013),/10.1016/j.cca.2013.11.003
-
攀登英语实验方案
攀登英语课题实验方案桃红坡明德小学一、本学期实验情况分析1、课题组的组成。本学期,我校的攀登英语课题组,依然是由本校校长任课题组长…
-
小学低段学生数学审题能力培养的实践与研究实验方案
《小学低段学生数学审题能力培养的实践与研究》实验方案一、课题提出新课程背景下解决实际问题的题目和传统算术应用题相比,一改传统应用题…
-
生脉饮对小鼠酒精中毒的解救实验方案
探讨生脉注射液对小鼠酒精中毒的解救作者:立题依据:生脉饮是在我国千年古方“生脉散”(含人参、麦冬、五味子)基础上用现代科学技术研制…
-
(俞善群)“三步走”书法教学研究课题实验方案Microsoft Word 文档
“三步走”书法教学研究课题实验方案一、研究的背景和意义很长一段时间以来,中小学由于受应试教育思想的影响,教师对书法指导意识弱化,加…
-
创造性使用数学教材的研究实验方案
《创造性使用数学教材的研究》实验方案威海市码头小学一、课题提出的背景我国的教育家叶圣陶曾说过:“教材无非是个例子,凭借这个例子使学…
-
科室_质控总结
针灸科20xx年质控总结医疗质量管理是医院管理的核心,提高医疗质量是管理科室根本目的。医疗质量是医院的生命线,医疗水平的高低、医疗…
-
实验室质量控制方案
实验室质量控制方案1定义11质量保证是环境监测过程的全面质量管理包含了保证环境监测数据准确可靠的全部活动和措施12质量控制指以满足…
-
浅谈实验室管理的质量控制工作
浅谈实验室管理的质量控制工作杨景江云南省水文水资源局西双版纳分局666100摘要实验室质量控制工作是实验室检测工作顺利进行的强有力…
-
实验室质量控制
理化检测实验室质量控制浙江康洁消防检测有限公司邢国康管理体系的建立实验室应建立实施和维持与其活动范围相适应的管理体系1建立依据1I…
-
实验室质量控制试题
实验室质量管理培训班考试题单位姓名职称一单选题每题2分1在免疫应答中最早产生的抗体是AIgABIgMCIgGDIgEEIgD2所有…