高中化学必修二方程式总结(全面精确排版)
高中化学必修二化学方程式汇总
1、Li与O2反应(点燃):
2、K与H2O的反应:
3、卤素单质氟与氢气反应:
4、卤素单质氯与氢气反应:
5、卤素单质溴与氢气反应:
6、卤素单质碘与氢气反应:
7、氯水与饱和溴化钠溶液反应:
8、氯水与饱和碘化钠溶液反应:
9、溴水与碘化钠溶液反应:
10、Mg与H2O反应:
11、用电子式表示氯化氢的形成过程:
12、用电子式表示氯化钠的形成过程:
13、1、Ba(OH)2?8H2O与NH4Cl的反应 :
14、典型的原电池(Zn-Cu原电池)电极反应式
负极(锌):
正极(铜):
总反应离子方程式:
15、氢气氧气燃料电池(KOH溶液作电解质溶液)
负极:
正极:
总反应方程式:
16、氢气氧气燃料电池(稀硫酸作电解质溶液)
负极:
正极:
总反应方程式:
17、甲烷氧气燃料电池(KOH溶液作电解质溶液)
负极:
正极:
总反应方程式:
18、甲醇氧气燃料电池(KOH溶液作电解质溶液)
负极:
正极:
总反应方程式:
19、镁铝稀硫酸电池
负极:
正极:
总反应方程式:
20、镁铝氢氧化钠溶液电池
负极:
正极:
总反应方程式:
21、铜铝浓硝酸溶液电池
负极:
正极:
总反应方程式:
22、铅蓄电池(以Pb和PbO2为电极材料,浓硫酸为电解质溶液)
负极:
正极:
总反应方程式:
23、H2O2在催化剂作用下受热分解:
24、高炉炼铁涉及的反应(教材50页):
25、甲烷与O2的反应:
26、甲烷与Cl2的反应(生成四种不同的取代物):
27、乙烯与氧气点燃条件下的反应:
28、乙烯与溴的四氯化碳溶液的反应:
29、乙烯生成聚乙烯的反应:
30、丙烯生成聚丙烯的反应:
31、乙烯与氢气的反应:
32、乙烯与氯化氢的反应:
33、乙烯与氯气的反应:
34、乙烯与水的反应:
35、氯乙烯制聚氯乙烯的反应:
36、苯与O2的反应:
37、苯与Br2的反应:
38、苯与浓硝酸的反应:
39、苯与氢气的反应:
40、乙醇与金属钠的反应:
41、乙醇的燃烧:
42、乙醇的催化氧化反应:
43、乙醇在常温下的氧化反应:CH3CH2OH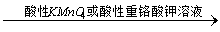 CH3COOH
CH3COOH
44、用乙酸来除去水垢的反应:
45、乙酸与金属钠的反应:
46、乙酸与乙醇的反应:
47、乙酸丙酯在酸性条件下的水解反应:
48、甲酸乙酯在氢氧化钠溶液中的反应
49、蔗糖水解反应:
50、淀粉(纤维素)水解反应:
51、硬脂酸甘油酯在酸性条件下的水解:
52、硬脂酸甘油酯在氢氧化钠溶液中的反应(皂化反应):
53、HgO受热分解:
54、Ag2O受热分解:
55、CO还原Fe2O3:
56、Al 还原Fe2O3(铝热反应):
57、Al 还原Fe3O4(铝热反应):
58、电解NaCl:
59、电解MgCl2:
60、电解Al2O3:
61、煤气化的反应:
62、乙二酸与乙二醇反应生成高聚物的方程:
63、乳酸发生反应生成高聚物的方程:
64、葡萄糖的结构简式:
果糖的结构简式:
书写出下列物质的电子式
1、Cl- 2、S2- 3、O22-
4、NH4+ 5、OH-
6、CaCl2 所含化学键有:
7、NaOH 所含化学键有:
8、Na2O2 所含化学键有:
9、H2O2 所含化学键有:
10、CO2 所含化学键有:
11、HCl 所含化学键有:
12、H2O 所含化学键有:
13、NH3 所含化学键有:
14、CH4 所含化学键有:
15、NH4Cl 所含化学键有:
16、HClO 所含化学键有:
第二篇:高一化学必修1方程式总结
必修一化学方程式及离子方程式小结
1、硫酸根离子的检验:
BaCl2 + Na2SO4 = BaSO4↓+ 2NaCl SO42- + Ba2+ == BaSO4↓ 2、碳酸根离子的检验:
CaCl2 + Na2CO3 = CaCO3↓ + 2NaCl CO32- + Ca2+== CaCO3↓ 3、碳酸钠与盐酸反应:
Na2CO3 + 2HCl = 2NaCl + H2O + CO2↑ CO32- + 2H+== CO2↑+ H2O 4、木炭还原氧化铜: 2CuO + C高温2↑ 5、钠与非金属单质反应:
点燃点燃
4Na+O2=2Na2O 2Na+O2 == Na2O2 Cl2 +2Na == 2NaCl 6、钠与水反应:
2Na+2H2O=2NaOH+H2↑ 2Na + 2H2O == 2Na+ + 2OH- + H2↑ 7、氧化钠的主要化学性质:2Na2O+O2 == 2Na2O2 Na2O+H2O=2NaOH Na2O+CO2=Na2CO3
Na2O+SO3=Na2SO4 Na2O+2HCl=2NaCl+H2O
8、过氧化钠的主要反应:
2Na2O2+2H2O = 4NaOH+O2↑;2Na2O2+2CO2 = 2Na2CO3+O2 Na2O2+H2SO4(冷、稀)= Na2SO4+H2O2 9、氯气的主要化学性质:
点燃
Cl2 +H2 光照2 +2P == 2PCl3 点燃点燃
Cl2 +PCl3 == PCl5 3Cl2 +2Fe == 2FeCl3 点燃点燃
Cl2 +2Na == 2NaCl Cl2+Cu == CuCl2 Cl2 +2FeCl2 =2FeCl3 Cl2 + 2Fe2+ == 2Fe3+ + 2Cl- Cl2 + 2NaBr = Br2 + 2NaCl Cl2 + 2Br- = Br2 + 2Cl- Cl2 + 2KI =2KCl + I2 Cl2 + 2I- == 2Cl- + I2 Cl2+H2O=HCl +HClO Cl2 + H2O == Cl- + H+ + HClO 2HClO == 2HCl + O2↑
Cl2+SO2 +2H2O=H2SO4 +2HCl Cl2 + SO2 + 2H2O == 2Cl- + SO42-+ 4H+ Cl2+2NaOH=NaCl+NaClO+H2O Cl2 + 2OH- == Cl- + ClO- + H2O 2Cl2+2Ca(OH) 2=CaCl2+Ca(ClO) 2+2H2O 2Ca(OH)2 +2Cl2 =2Ca2++2ClO-+2Cl-+2H2O Ca(ClO)2+CO2+H2O=CaCO3↓+2HClO Ca2++2ClO- + CO2 + H2O =CaCO3↓+ 2HClO 10、铁及其化合物的主要化学性质:
点燃点燃
2Fe + 3Cl2 == 2FeCl3 3Fe + 2O2 == Fe3O4 Fe + S △ FeS 3Fe+4H2O(g) 高温 Fe3O4+4H2 Fe+2HCl=FeCl2+H2↑ Fe+2H+ = Fe2+ + H2↑ Fe + CuSO4 = FeSO4 + Cu Fe + Cu2+ = Fe2+ + Cu
4Fe(OH)2 + O2 + 2H2O == 4 Fe(OH) 3 2Fe(OH)3 △ Fe2O3 + 3H2O 2FeCl2 + Cl2=2FeCl3 2FeCl3+Fe=3FeCl2 2FeCl3+Cu=2FeCl2+CuCl2
FeCl3 + 3KSCN == Fe(SCN)3 + 3KCl
Fe3+ + 3SCN- == Fe(SCN)3(红色溶液) Fe + 2Fe3+ == 3Fe2+ 2Fe3+ + Cu == 2Fe2+ + Cu2+ Fe3+ + 3OH- == Fe(OH)3↓ 11、碳及其化合物的主要化学性质: 点燃
2C + O2(少量) == 2CO
点燃
C + O2 (足量) == CO2
C + CO2 高温 2O高温2 (生成水煤气) C+2H2SO4(浓) == CO2↑+2SO2↑+2H2O C +4HNO3(浓) == CO2↑+4NO2↑+2H2O
点燃
2CO+O2 == 2CO2 CO+CuO高温Cu+CO2 3CO+Fe2O3高温 2 CO2+H2O=H2CO3 CO2+Ca(OH)2 (过量)=CaCO3↓+H2O Ca2+ + 2OH- + CO2 == CaCO3↓ + H2O 2CO2 (过量)+Ca(OH)2=Ca(HCO3)2
CO2 + 2OH- == CO32- + H2O
CO2 + OH- == HCO3-
CO2+NH3+NaCl+H2O=NaHCO3↓+NH4Cl(侯氏制碱法)
12、氮气、氨气及硝酸的主要化学性质: N2+3H2高温,高压,催化剂 2NH3 N2+O2 一定条件2NO
点燃
N2+3Mg== Mg3N2 2NO+O2=2NO2 3NO2+H2O=2HNO3+NO 4NH3+5O2一定条件4NO+6H2O
NH3+HCl=NH4Cl(白烟) NH3 + H2O == NH3.H2O == NH4+ + OH- NH4HCO3 △ NH3↑+H2O+CO2↑ NH4Cl△ NH3+HCl
2NH4Cl+Ca(OH)2 ==CaCl2+ NH3↑+H2O NH4+ + OH- == NH3↑+ H2O 4HNO3光照 4NO2↑+O2↑+2H2O 4HNO3(浓)+C == CO2↑+4NO2↑+2H2O 4HNO3+Cu=Cu(NO3)2+2NO2↑+2H2O 2NO3- + Cu +4H+= Cu2++2NO2↑+ 2H2O 8HNO3+3Cu=3Cu(NO3)2+2NO↑+4H2O 2NO3- + 3Cu + 8H+ == 3Cu2+ + 2NO↑+ 4H2O 13、硫及其化合物的化学性质:
点燃
S+Fe △ FeS S+2Cu △ Cu2S S+O2== SO2 2SO2+O2高温2SO3 3S + 6NaOH == 2Na2S + Na2SO3 + 3H2O 3S + 6OH- == 2S2- + SO32- + 3H2O SO2 + 2H2S=3S↓+2H2O SO2+H2O=H2SO3
2NaOH+SO2 (少量)=Na2SO3+H2O SO2 + 2OH == SO3 + H2O NaOH+SO2(足量)=NaHSO3 SO2 + OH- == HSO3- 2H2SO4(浓)+C == CO2↑ +2SO2↑+2H2O 2H2SO4(浓)+Cu CuSO4+SO2↑+2H2O
Na2SO3+H2SO4 = Na2SO4+ SO2↑+ H2O SO32- + 2H+== SO2↑+ H2O 14、铝及其化合物主要化学性质:
点燃
4Al+3O2 ==2Al2O3(纯氧) 2Al+Fe2O2O3+2Fe 2Al+3H2SO4=Al2(SO4)3+3H2↑ 2Al + 6H+ = 2Al3+ + 3H2↑
2Al+2NaOH+2H2O=2NaAlO2+3H2↑或 2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2↑ 2Al+2OH-+6H2O=2[Al(OH)4]-+3H2↑
Al2O3+3H2SO4=Al2(SO4)3+3H2O Al2O3+6H+=2Al3++3H2O
Al2O3+2NaOH+3H2O=2Na[Al(OH)4] Al2O3+2OH—+3H2O=2[Al(OH)4]- 2 Al2O3(熔融)通电 3O2↑ + 4Al 2Al(OH)3 高温Al2O3+3H2O Al(OH)3+3HCl=AlCl3+3H2O Al(OH)3+3H+=Al3++3H2O
-2-
Al(OH)3+NaOH=Na[Al(OH)4] Al(OH)3+OH—=[Al(OH)4]- AlCl3+3NaOH=Al(OH)3↓+3NaCl Al3+ + 3OH- == Al(OH)3↓
AlCl3+3NH3.H2O=Al(OH)3↓+3NH4Cl Al3+ + 3NH3.H2O == Al(OH)3↓+ 3NH4+ AlCl3+3NaHCO3=Al(OH)3↓+3CO2↑ Al3+ + 3HCO3- == Al(OH)3↓ + 3CO2↑ 15、硅及其化合物主要化学性质:
Si(粗)+2Cl2高温SiCl4 SiCl4 +2H2 高温 Si(纯)+4HCl Si(粉)+O2 高温SiO2
Si+2NaOH+H2O=Na2SiO3+2H2 Si+ 2OH- + H2O == SiO32- + 2H2↑ 2C+SiO2制得粗硅) 4HF+SiO2=SiF4+2H2O
SiO2+CaO高温CaSiO3
SiO2+2NaOH=Na2SiO3+H2O (常温下强碱缓慢腐蚀玻璃) SiO2+Na2CO3 == Na2SiO3+CO2 SiO2+CaCO3 高温 CaSiO3+CO2↑ 2NaOH+SiO2=Na2SiO3+H2O SiO2 + 2OH- == SiO32- + H2O Na2SiO3 + CO2 + H2O == H2SiO3↓+ Na2CO3 SiO32- + CO2 + H2O == H2SiO3↓+ CO32- 16、镁、铜等单质及化合物的性质:
点燃点燃点燃
2Mg+O2 ==2MgO Mg + Cl2== MgCl2 2Mg +CO2 ==2MgO+C Mg + 2H2O △ 2Mg(OH) 2↓ + H2↑
Mg + H2SO4 = MgSO4 + H2↑ Mg + 2H+ == Mg2+ + H2↑ MgO + 2HCl = MgCl2 +H2O MgO + 2H+ == Mg2+ + H2O Mg(OH)2 + 2HCl = MgCl2 +2H2O Mg(OH)2 + 2H+ = Mg2+ + 2H2O MgCl2+2NaOH=Mg(OH)2↓+2NaCl Mg2+ + 2OH- = Mg(OH)2↓ 点燃
2Cu +O2 △ △ Cu2S Cu+ Cl2 == CuCl2 CuO+H2SO4=CuSO4+H2O CuO + 2H+ == Cu2+ + H2O Cu(OH)2+H2SO4=CuSO4+2H2O Cu(OH)2 + 2H+ == Cu2+ + 2H2O Cu(OH)2 △ 2O Cu2(OH)2CO3 △ 2CuO + CO2↑+ H2O CuCl2+2NaOH=Cu(OH)2↓+2NaCl Cu2+ + 2OH- == Cu(OH)2↓ CuSO4+H2S=CuS↓+H2SO4 Cu2+ +H2S=CuS↓+2H+
-
必修二有机化学方程式归纳+答案
必修二有机化学方程式归纳班级姓名一写出下列反应的方程式除燃烧外有机物必须用结构简式表示1甲烷与氯气混合光照光照光照CH4Cl2CH…
-
高中化学人教版必修二有机化合物化学方程式总结
高中化学人教版必修二有机化合物单元知识点总结甲烷燃烧CH4+2O2→CO2+2H2O(条件为点燃)甲烷隔绝空气高温分解甲烷分解很复…
-
高中化学必修二方程式总结
高中化学人教版必修二相关化学方程式汇总第一章物质结构元素周期律1Li与O2反应点燃4LiO22Li2ONa与O2反应点燃2NaO2…
-
高中化学必修2化学方程式总汇
高中化学必修2化学方程式总汇(知识点总结)第一章物质结构元素周期律1、Li与O2反应(点燃)P6Na与O2反应(点燃)P6Na与H…
-
化学必修二化学方程式总结
必修二化学方程式第一章物质结构元素周期律1Li与O2反应点燃4LiO2Na与O2反应点燃2NaO22Li2ONa2O2Na与H2O…
-
高中化学人教版必修二有机化合物化学方程式总结
高中化学人教版必修二有机化合物单元知识点总结甲烷燃烧CH4+2O2→CO2+2H2O(条件为点燃)甲烷隔绝空气高温分解甲烷分解很复…
-
高中必修二化学方程式汇总
必修二第一章物质结构元素周期律1、Li与O2反应(点燃):4Li+O22Li2ONa与O2反应(点燃):2Na+O2Na2O2Na…
-
高中化学必修二知识点总结
高中化学必修二知识点总结第一章物质结构元素周期律1原子结构如的质子数与质量数中子数电子数之间的关系2元素周期表和周期律1元素周期表…
-
高一化学必修二知识点总结
高一化学必修二知识点总结20xx6251548提问者麟鼠度高一化学必修二知识点总结20xx6251612最佳答案1原子半径1除第1…
-
高中化学必修二知识点总结1
物质结构元素周期律周期同一横行周期序数电子层数类别周期序数起止元素包括元素种数核外电子层数短周期1HHe212LiNe823NaA…
-
高一化学必修二有机物知识点总结
必修二有机物一、有机物的概念1、定义:含有碳元素的化合物为有机物(碳的氧化物、碳酸、碳酸盐、碳的金属化合物等除外)2、特性①种类多…